Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response
- PMID: 31092698
- PMCID: PMC6549090
- DOI: 10.1042/BSR20190805
Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response
Abstract
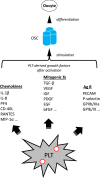
In clinical infertility practice, one intractable problem is low (or absent) ovarian reserve which in turn reflects the natural oocyte depletion associated with advancing maternal age. The number of available eggs has been generally thought to be finite and strictly limited, an entrenched and largely unchallenged tenet dating back more than 50 years. In the past decade, it has been suggested that renewable ovarian germline stem cells (GSCs) exist in adults, and that such cells may be utilized as an oocyte source for women seeking to extend fertility. Currently, the issue of whether mammalian females possess such a population of renewable GSCs remains unsettled. The topic is complex and even agreement on a definitive approach to verify the process of 'ovarian rescue' or 're-potentiation' has been elusive. Similarities have been noted between wound healing and ovarian tissue repair following capsule rupture at ovulation. In addition, molecular signaling events which might be necessary to reverse the effects of reproductive ageing seem congruent with changes occurring in tissue injury responses elsewhere. Recently, clinical experience with such a technique based on autologous activated platelet-rich plasma (PRP) treatment of the adult human ovary has been reported. This review summarizes the present state of understanding of the interaction of platelet-derived growth factors with adult ovarian tissue, and the outcome of human reproductive potential following PRP treatment.
Keywords: aging; fertility; menopause; ovary; reproduction.
© 2019 The Author(s).
Conflict of interest statement
E.S.S. holds a provisional U.S. patent for process and treatment using ovarian PRP.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

