Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy
- PMID: 31092823
- PMCID: PMC6520358
- DOI: 10.1038/s41467-019-10195-z
Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy
Abstract
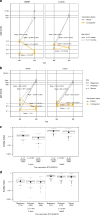
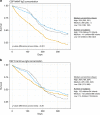
RTS,S/AS01E has been tested in a phase 3 malaria vaccine study with partial efficacy in African children and infants. In a cohort of 1028 subjects from one low (Bagomoyo) and two high (Nanoro, Kintampo) malaria transmission sites, we analysed IgG plasma/serum concentration and avidity to CSP (NANP-repeat and C-terminal domains) after a 3-dose vaccination against time to clinical malaria events during 12-months. Here we report that RTS,S/AS01E induces substantial increases in IgG levels from pre- to post-vaccination (p < 0.001), higher in NANP than C-terminus (2855 vs 1297 proportional change between means), and higher concentrations and avidities in children than infants (p < 0.001). Baseline CSP IgG levels are elevated in malaria cases than controls (p < 0.001). Both, IgG magnitude to NANP (hazard ratio [95% confidence interval] 0.61 [0.48-0.76]) and avidity to C-terminus (0.07 [0.05-0.90]) post-vaccination are significantly associated with vaccine efficacy. IgG avidity to the C-terminus emerges as a significant contributor to RTS,S/AS01E-mediated protection.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

