Interactions between Karlodinium veneficum and Prorocentrum donghaiense from the East China Sea
- PMID: 31093028
- PMCID: PMC6512812
- DOI: 10.1016/j.hal.2015.08.004
Interactions between Karlodinium veneficum and Prorocentrum donghaiense from the East China Sea
Abstract
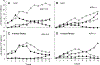
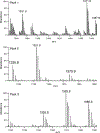
The dinoflagellate Prorocentrum donghaiense is a dominant harmful algal bloom (HAB) species on the East China Sea (ECS) coast. The co-occurrence of Karlodinium veneficum with P. donghaiense is often observed and can later develop into dense blooms. However, the role of K. veneficum in P. donghaiense population dynamics is unknown. In the current study, three K. veneficum (GM1, GM2, and GM3) strains were isolated from the ECS with one (GM1) from a mixed, dense bloom of P. donghaiense and other HAB species. All three isolates had identical ITS sequences that were concordant with the species designation. Unique karlotoxin congeners were isolated from one strain (GM2). The sterol compositions of P. donghaiense and K. veneficum were consistent with sensitivity to karlotoxin in the former and insensitivity in the latter. Additional experimentation showed that: (1)in monocultures, higher growth rate of P. donghaiense than K. veneficum is observed in nutrient-enriched and nutrient-depleted media. In co-cultures, the growth of P. donghaiense is inhibited; (2) feeding on P. donghaiense by K. veneficum is clearly demonstrated by fluorescent dye tracking; and (3) the isolated karlotoxin is lethal to P. donghaiense in a concentration-dependent manner. From these studies we propose that K. veneficum may play a negative role in P. donghaiense bloom maintenance and that P. donghaiense may in turn be a bloom initiator as a prey item for K. veneficum.
Keywords: Co-culture; Karlodinium veneficum; Karlotoxin; Phagotrophy; Prorocentrum donghaiense; Sterol.
Figures


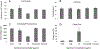
References
-
- Adolf JE, Bachvaroff T, Krupatkina DN, Nonogaki H, Brown PJP, Lewitus AJ, 2006. Species specificity and potential roles of Karlodinium micrum toxin. Afr. J. Med. Sci. 28, 415–421.
-
- Adolf JE, Krupatkina D, Bachvaroff T, Place AR, 2007. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum. Harmful Algae 6,400–412.
-
- Bachvaroff TR, Adolf JE, Squier AH, Harvey HR, Place AR, 2008. Characterization and quantification of karlotoxins by liquid chromatography - mass spectrometry. Harmful Algae 7, 473–484.
-
- Bligh EG, Dyer WJ, 1959. A rapid method of total lipid extraction and purification. Can. Biochem. Physiol. 37, 911–917. - PubMed
-
- Barrett SM, Volkman JK, Dunstan GA, LeRoi JM, 1995. Sterols of 14 species of marine diatoms. J. Phycol. 31, 360–369.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
