Efficiently Capturing Mitochondria-Targeted Constituents with Hepatoprotective Activity from Medicinal Herbs
- PMID: 31093314
- PMCID: PMC6481013
- DOI: 10.1155/2019/4353791
Efficiently Capturing Mitochondria-Targeted Constituents with Hepatoprotective Activity from Medicinal Herbs
Abstract
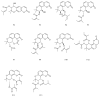
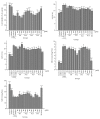
Targeting mitochondria as a hepatic-protective strategy has gained attention, because of their important roles in energy production, adjustment of apoptosis, and generation of reactive oxygen species. To promote the discovery of natural mitochondria-targeted hepatic-protectants, we established a hepatocellular mitochondria-based capturing method by coupling affinity ultrafiltration with liquid chromatography/mass spectrometry (LC/MS), which is suitable for identifying mitochondrial ligands from medicinal herbs (MHs). After evaluating the feasibility of the method, it was applied for capturing mitochondria-targeting constituents from Peucedani Radix extract. A total of 10 active compounds were identified by LC/MS, all of which were newly identified mitochondrial ligands. The mitochondria-remedying activity of 4 of the 10 hits was confirmed by pharmacological tests in vitro. Additionally, the hepatic-protective abilities of 4 hits were verified in both carbon tetrachloride-damaged liver L02 cells and mice. These results indicated that the method could be used for identifying hepatic mitochondria-targeting constituents in MHs, which might be beneficial for hepatic-protective development.
Figures








Similar articles
-
Identification of Mitochondrial Ligands with Hepatoprotective Activity from Notopterygii Rhizoma et Radix Using Affinity Ultrafiltration/Liquid Chromatography/Mass Spectrometry.Biomed Res Int. 2019 Dec 16;2019:5729263. doi: 10.1155/2019/5729263. eCollection 2019. Biomed Res Int. 2019. PMID: 31950043 Free PMC article.
-
Development of a mitochondria-based centrifugal ultrafiltration/liquid chromatography/mass spectrometry method for screening mitochondria-targeted bioactive constituents from complex matrixes: Herbal medicines as a case study.J Chromatogr A. 2015 Sep 25;1413:33-46. doi: 10.1016/j.chroma.2015.08.014. Epub 2015 Aug 12. J Chromatogr A. 2015. PMID: 26306914
-
Protective effects of Akebia saponin D against rotenone-induced hepatic mitochondria dysfunction.J Pharmacol Sci. 2014;126(3):243-52. doi: 10.1254/jphs.14135fp. Epub 2014 Oct 15. J Pharmacol Sci. 2014. PMID: 25319614
-
Hepatoprotective properties of extensively studied medicinal plant active constituents: possible common mechanisms.Pharm Biol. 2015 Jun;53(6):781-91. doi: 10.3109/13880209.2014.950387. Epub 2014 Dec 9. Pharm Biol. 2015. PMID: 25489628 Review.
-
Escalation of liver malfunctioning: A step toward Herbal Awareness.J Ethnopharmacol. 2018 Apr 24;216:104-119. doi: 10.1016/j.jep.2018.01.002. Epub 2018 Jan 6. J Ethnopharmacol. 2018. PMID: 29309862 Review.
Cited by
-
Identification of Mitochondrial Ligands with Hepatoprotective Activity from Notopterygii Rhizoma et Radix Using Affinity Ultrafiltration/Liquid Chromatography/Mass Spectrometry.Biomed Res Int. 2019 Dec 16;2019:5729263. doi: 10.1155/2019/5729263. eCollection 2019. Biomed Res Int. 2019. PMID: 31950043 Free PMC article.
References
-
- McGill M.-R., Sharpe M.-R., Williams C.-D., Taha M., Curry S.-C., Jaeschke H. The mechanism underlying acetaminophen -induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. Journal of Clinical Investigation. 2012;122(4):1574–1583. doi: 10.1172/JCI59755. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

