The cylindromatosis (CYLD) gene and head and neck tumorigenesis
- PMID: 31093340
- PMCID: PMC6460526
- DOI: 10.1186/s41199-016-0012-y
The cylindromatosis (CYLD) gene and head and neck tumorigenesis
Abstract
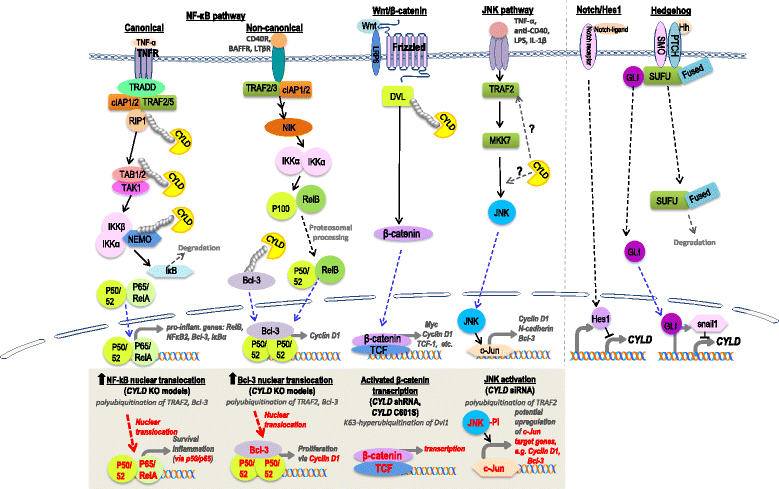
Germline CYLD mutation is associated with the development of a rare inheritable syndrome, called the CYLD cutaneous syndrome. Patients with this syndrome are distinctly presented with multiple tumors in the head and neck region, which can grow in size and number over time. Some of these benign head and neck tumors can turn into malignancies in some individuals. CYLD has been identified to be the only tumor suppressor gene reported to be associated with this syndrome thus far. Here, we summarize all reported CYLD germline mutations associated with this syndrome, as well as the reported paired somatic CYLD mutations of the developed tumors. Interestingly, whole-exome sequencing (WES) studies of multiple cancer types also revealed CYLD mutations in many human malignancies, including head and neck cancers and several epithelial cancers. Currently, the role of CYLD mutations in head and neck carcinogenesis and other cancers is poorly defined. We hope that this timely review of recent findings on CYLD genetics and animal models for oncogenesis can provide important insights into the mechanism of head and neck tumorigenesis.
Keywords: Brooke-Spiegler Syndrome (BSS); Cylindromatosis (CYLD); Deubiquitinating (DUB); Familial Cylindromatosis (FC); Head and Neck Cancer; Multiple Familial Trichoepithelioma (MFT1); Nuclear Factor-kB (NF-kB); TNF-receptor associated factor (TRAF) proteins; The CYLD cutaneous syndrome; Turban Tumor Syndrome; and B-cell lymphoma 3 (Bcl-3); tumorigenesis.
Conflict of interest statement
VWYL served as a Consultant for Novartis Pharmaceuticals (HK) Ltd. All other authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
