Pharmacokinetics of tenofovir monoester and association with intracellular tenofovir diphosphate following single-dose tenofovir disoproxil fumarate
- PMID: 31093649
- PMCID: PMC8420957
- DOI: 10.1093/jac/dkz187
Pharmacokinetics of tenofovir monoester and association with intracellular tenofovir diphosphate following single-dose tenofovir disoproxil fumarate
Abstract
Background: Tenofovir monoester is a relatively lipophilic intermediate formed during the hydrolysis of tenofovir disoproxil to tenofovir. Its clinical pharmacokinetic profile and influence on the cellular pharmacology of tenofovir diphosphate have not been reported.
Methods: Plasma, PBMC and dried blood spots (DBS) were obtained from HIV-uninfected adults participating in a randomized, cross-over bioequivalence study of single-dose tenofovir disoproxil fumarate (TDF)/emtricitabine unencapsulated or encapsulated with a Proteus® ingestible sensor. Plasma pharmacokinetics of tenofovir monoester and tenofovir were characterized using non-compartmental methods. Relationships with tenofovir diphosphate in DBS and PBMC were examined using mixed-effects models.
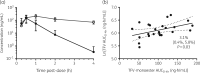
Results: Samples were available from 24 participants (13 female; 19 white, 3 black, 2 Hispanic). Tenofovir monoester appeared rapidly with a median (range) Tmax of 0.5 h (0.25-2) followed by a rapid monophasic decline with a geometric mean (coefficient of variation) t½ of 26 min (31.0%). Tenofovir monoester Cmax was 131.6 ng/mL (69.8%) and AUC0-4 was 93.3 ng·h/mL (47.9%). The corresponding values for plasma tenofovir were 222.2 ng/mL (37.1%) and 448.1 ng·h/mL (30.0%). Tenofovir monoester AUC0-∞ (but not tenofovir AUC0-∞) was a significant predictor of tenofovir diphosphate in both PBMC (P = 0.015) and DBS (P = 0.005), increasing by 3.8% (95% CI 0.8%-6.8%) and 4.3% (95% CI 1.5%-7.2%), respectively, for every 10 ng·h/mL increase in tenofovir monoester.
Conclusions: Tenofovir monoester Cmax and AUC0-4 were 59.2% and 20.6% of corresponding plasma tenofovir concentrations. Tenofovir monoester was significantly associated with intracellular tenofovir diphosphate concentrations in PBMC and DBS, whereas tenofovir concentrations were not. Tenofovir monoester likely facilitates cell loading, thereby increasing tenofovir diphosphate exposures in vivo.
© The Author(s) 2019. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Murphy RA, Valentovic MA.. Factors contributing to the antiviral effectiveness of tenofovir. J Pharmacol Exp Ther 2017; 363: 156–63. - PubMed
-
- Shaw JP, Sueoko CM, Oliyai R. et al.Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm Res 1997; 14: 1824–9. - PubMed
-
- Custodio J, Garner W, Callebaut C.. The pharmacokinetics of tenofovir and tenofovir diphosphate following administration of tenofovir alafenamide versus tenofovir disoproxil fumarate. In: 16th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, Washington, DC, USA, Abstract 6.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

