Brainstem Functional Connectivity Disturbances in Epilepsy may Recover After Successful Surgery
- PMID: 31093673
- PMCID: PMC7308661
- DOI: 10.1093/neuros/nyz128
Brainstem Functional Connectivity Disturbances in Epilepsy may Recover After Successful Surgery
Abstract
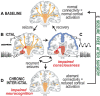
Background: Focal seizures in temporal lobe epilepsy (TLE) are associated with widespread brain network perturbations and neurocognitive problems.
Objective: To determine whether brainstem connectivity disturbances improve with successful epilepsy surgery, as recent work has demonstrated decreased brainstem connectivity in TLE that is related to disease severity and neurocognitive profile.
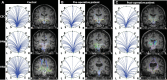
Methods: We evaluated 15 adult TLE patients before and after (>1 yr; mean, 3.4 yr) surgery, and 15 matched control subjects using magnetic resonance imaging to measure functional and structural connectivity of ascending reticular activating system (ARAS) structures, including cuneiform/subcuneiform nuclei (CSC), pedunculopontine nucleus (PPN), and ventral tegmental area (VTA).
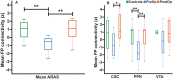
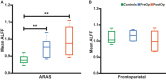
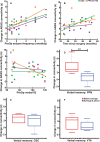
Results: TLE patients who achieved long-term postoperative seizure freedom (10 of 15) demonstrated increases in functional connectivity between ARAS structures and fronto-parietal-insular neocortex compared to preoperative baseline (P = .01, Kruskal-Wallis), with postoperative connectivity patterns resembling controls' connectivity. No functional connectivity changes were detected in 5 patients with persistent seizures after surgery (P = .9, Kruskal-Wallis). Among seizure-free postoperative patients, larger increases in CSC, PPN, and VTA functional connectivity were observed in individuals with more frequent seizures before surgery (P < .05 for each, Spearman's rho). Larger postoperative increases in PPN functional connectivity were seen in patients with lower baseline verbal IQ (P = .03, Spearman's rho) or verbal memory (P = .04, Mann-Whitney U). No changes in ARAS structural connectivity were detected after successful surgery.
Conclusion: ARAS functional connectivity disturbances are present in TLE but may recover after successful epilepsy surgery. Larger increases in postoperative connectivity may be seen in individuals with more severe disease at baseline.
Keywords: Brainstem; Epilepsy surgery; Functional connectivity; Postoperative; Temporal lobe epilepsy.
Copyright © 2019 by the Congress of Neurological Surgeons.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

