International Expert Consensus on a Cell Therapy Communication Tool: DOSES
- PMID: 31094982
- PMCID: PMC7292498
- DOI: 10.2106/JBJS.18.00915
International Expert Consensus on a Cell Therapy Communication Tool: DOSES
Abstract
Background: The lack of a standardized system for describing cell therapies acts as a barrier to advancement in clinical and basic research and practice. The aim of this study was to establish an international expert consensus on strategies to improve standardization and transparency when describing cell therapies. The secondary aim was to develop a consensus among experts on the contents of a standardized tool for describing cell therapies.
Methods: The need for expert consensus on strategies to improve cell therapy communication was confirmed at the American Academy of Orthopaedic Surgeons/National Institutes of Health Optimizing Clinical Use of Biologics Symposium in 2018. A working group of 6 experts convened an international consensus process involving clinicians and basic scientists using validated Delphi methodology. This iterative process was used to define statements on communication of cell therapies and develop a standardized tool for describing cell therapies.
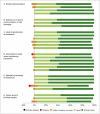
Results: Thirty-four experts completed 3 rounds survey with use of the Delphi process. After 3 rounds, 27 statements relating to existing nomenclature, solutions to improve communication, ideal characteristics of a framework, mandatory elements of a new framework, and future work to facilitate application reached consensus with >80% agreement and <5% disagreement. Consensus was reached on the contents of a tool for improving standardization and transparency when describing cell therapies. This tool, dubbed "DOSES," is based on the reporting of 5 core items: donor (i.e., autologous, allogeneic, xenogeneic), origin of tissue, separation from other cell types/preparation method, exhibited cell characteristics associated with behavior, and the site of delivery.
Conclusions: This study has established expert consensus on the communication of cell therapies. The DOSES tool has been developed to improve standardization and transparency in describing cell therapies.
Clinical relevance: The DOSES tool for describing cell therapies can be utilized by researchers, clinicians, regulators, and industry professionals to improve standardization and transparency when describing cell therapies. The use of this tool may allow clinicians and patients to better understand the characteristics of current and future cell preparations.
Figures

Comment in
-
A Call for Standardization in Cell Therapy Studies: Commentary on an article by Iain R. Murray, BMedSci(Hons), MRCS, MFSEM, PhD, et al.: "International Expert Consensus on a Cell Therapy Communication Tool: DOSES".J Bone Joint Surg Am. 2019 May 15;101(10):e47. doi: 10.2106/JBJS.19.00189. J Bone Joint Surg Am. 2019. PMID: 31094994 No abstract available.
References
-
- Piuzzi NS, Khlopas A, Sodhi N, Oak S, Sultan AA, Chughtai M, Mantripragada VP, Mont MA, Muschler GF. Cellular therapies in orthopedics: where are we? Surg Technol Int. 2017. December 22;31:359-64. - PubMed
-
- Boregowda SV, Booker CN, Phinney DG. Mesenchymal stem cells: the moniker fits the science. Stem Cells. 2018. January;36(1):7-10. Epub 2017 Oct 17. - PubMed
-
- Turner L. The US direct-to-consumer marketplace for autologous stem cell interventions. Perspect Biol Med. 2018;61(1):7-24. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

