Hybrid Closed-Loop Control Is Safe and Effective for People with Type 1 Diabetes Who Are at Moderate to High Risk for Hypoglycemia
- PMID: 31095423
- PMCID: PMC6551970
- DOI: 10.1089/dia.2019.0018
Hybrid Closed-Loop Control Is Safe and Effective for People with Type 1 Diabetes Who Are at Moderate to High Risk for Hypoglycemia
Abstract
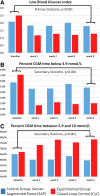
Background: Typically, closed-loop control (CLC) studies excluded patients with significant hypoglycemia. We evaluated the effectiveness of hybrid CLC (HCLC) versus sensor-augmented pump (SAP) in reducing hypoglycemia in this high-risk population. Methods: Forty-four subjects with type 1 diabetes, 25 women, 37 ± 2 years old, HbA1c 7.4% ± 0.2% (57 ± 1.5 mmol/mol), diabetes duration 19 ± 2 years, on insulin pump, were enrolled at the University of Virginia (N = 33) and Stanford University (N = 11). Eligibility: increased risk of hypoglycemia confirmed by 1 week of blinded continuous glucose monitor (CGM); randomized to 4 weeks of home use of either HCLC or SAP. Primary/secondary outcomes: risk for hypoglycemia measured by the low blood glucose index (LBGI)/CGM-based time in ranges. Results: Values reported: mean ± standard deviation. From baseline to the final week of study: LBGI decreased more on HCLC (2.51 ± 1.17 to 1.28 ± 0.5) than on SAP (2.1 ± 1.05 to 1.79 ± 0.98), P < 0.001; percent time below 70 mg/dL (3.9 mmol/L) decreased on HCLC (7.2% ± 5.3% to 2.0% ± 1.4%) but not on SAP (5.8% ± 4.7% to 4.8% ± 4.5%), P = 0.001; percent time within the target range 70-180 mg/dL (3.9-10 mmol/L) increased on HCLC (67.8% ± 13.5% to 78.2% ± 10%) but decreased on SAP (65.6% ± 12.9% to 59.6% ± 16.5%), P < 0.001; percent time above 180 mg/dL (10 mmol/L) decreased on HCLC (25.1% ± 15.3% to 19.8% ± 10.1%) but increased on SAP (28.6% ± 14.6% to 35.6% ± 17.6%), P = 0.009. Mean glucose did not change significantly on HCLC (144.9 ± 27.9 to 143.8 ± 14.4 mg/dL [8.1 ± 1.6 to 8.0 ± 0.8 mmol/L]) or SAP (152.5 ± 24.3 to 162.4 ± 28.2 [8.5 ± 1.4 to 9.0 ± 1.6]), P = ns. Conclusions: Compared with SAP therapy, HCLC reduced the risk and frequency of hypoglycemia, while improving time in target range and reducing hyperglycemia in people at moderate to high risk of hypoglycemia.
Keywords: Artificial pancreas; Closed-loop systems; Hypoglycemia; Type 1 diabetes.
Conflict of interest statement
S.M.A. has research support from Medtronic and has served as a consultant for Senseonics. B.A.B. is on medical advisory boards for Sanofi, Novo Nordisk, BD Biosciences, Unomedical, and Medtronic and has received research grant and/or material support from Medtronic, Dexcom, LifeScan, Insulet, Bayer, Unomedical, Tandem Diabetes Care, and Roche Diagnostics. M.D.B. has served on an advisory panel for Ascensia; had speaking engagements for Ascensia and Roche; received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Sanofi, and Tandem; and is receiving patent royalties managed by the University of Virginia Licensing and Ventures Group. J.L.R., C.L.B., C.A.W., M.C.O., P.K.C., L.J.H., R.S.K., L.M.N., S.E.L., and S.R.-D. have nothing to disclose. S.A.B. has received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Tandem, and Medtronic. T.T.L. has received research funding from Medtronic and Tandem and is currently employed by Insulet. B.P.K. has served on an advisory panel for Sanofi; had speaking engagements for Dexcom and Sanofi; received research grant/material support (to the University of Virginia) from Dexcom, Roche Diagnostics, Sanofi, and Tandem; and is receiving patent royalties managed by the University of Virginia Licensing and Ventures Group.
Figures

References
-
- The DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- Weinstock RS, Xing D, Maahs DM, et al. : Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

