Detoxification of host plant phenolic aglycones by the spruce budworm
- PMID: 31095557
- PMCID: PMC6522000
- DOI: 10.1371/journal.pone.0208288
Detoxification of host plant phenolic aglycones by the spruce budworm
Abstract
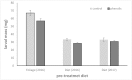
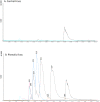
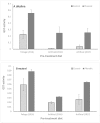
This study examines the post-ingestive fate of two host-plant derived small-molecule phenolics (the acetophenones piceol and pungenol) that have previously been shown to be toxic to the outbreaking forest pest, spruce budworm (Choristoneura fumiferana). We test first whether these compounds are transformed during passage through the midgut, and second whether the budworm upregulates activity of the detoxification enzyme glutathione-s-transferase (GST) in response to feeding on these compounds. Insects were reared on either foliage or artificial diet to the fourth instar, when they were transferred individually to one of two treatment diets, either control or phenolic-laced, for approximately 10 days, after which midguts were dissected out and used for Bradford soluble protein and GST enzyme activity analysis. Frass was collected and subjected to HPLC-DAD-MS. HPLC showed that the acetophenones do not autoxidize under midgut pH conditions, but that glucose- and glutathione- conjugates are present in the frass of insects fed the phenolic-laced diet. GST enzyme activity increases in insects fed the phenolic-laced diet, in both neutral pH and alkaline assays. These data show that the spruce budwom exhibits counter-adaptations to plant phenolics similar to those seen in angiosperm feeders, upregulating an important detoxifying enzyme (GST) and partially conjugating these acetophenones prior to elimination, but that these counter-measures are not totally effective at mitigating toxic effects of the ingested compounds in the context of our artifical-diet based laboratory experiment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Composition of the Spruce Budworm (Choristoneura fumiferana) Midgut Microbiota as Affected by Rearing Conditions.PLoS One. 2015 Dec 4;10(12):e0144077. doi: 10.1371/journal.pone.0144077. eCollection 2015. PLoS One. 2015. PMID: 26636571 Free PMC article.
-
Complementary roles of two classes of defense chemicals in white spruce against spruce budworm.Planta. 2024 Mar 29;259(5):105. doi: 10.1007/s00425-024-04383-5. Planta. 2024. PMID: 38551685
-
Genetic control and evolutionary potential of a constitutive resistance mechanism against the spruce budworm (Choristoneura fumiferana) in white spruce (Picea glauca).Heredity (Edinb). 2018 Aug;121(2):142-154. doi: 10.1038/s41437-018-0061-6. Epub 2018 Feb 17. Heredity (Edinb). 2018. PMID: 29453424 Free PMC article.
-
Paradigms in Eastern Spruce Budworm (Lepidoptera: Tortricidae) Population Ecology: A Century of Debate.Environ Entomol. 2016 Dec;45(6):1333-1342. doi: 10.1093/ee/nvw103. Epub 2016 Sep 1. Environ Entomol. 2016. PMID: 28028079 Review.
-
Surviving in a Frozen Forest: the Physiology of Eastern Spruce Budworm Overwintering.Physiology (Bethesda). 2021 May 1;36(3):174-182. doi: 10.1152/physiol.00037.2020. Physiology (Bethesda). 2021. PMID: 33904790 Review.
Cited by
-
Gene selection for studying frugivore-plant interactions: a review and an example using Queensland fruit fly in tomato.PeerJ. 2021 Aug 5;9:e11762. doi: 10.7717/peerj.11762. eCollection 2021. PeerJ. 2021. PMID: 34434644 Free PMC article.
-
Plant Allelochemicals as Sources of Insecticides.Insects. 2021 Feb 24;12(3):189. doi: 10.3390/insects12030189. Insects. 2021. PMID: 33668349 Free PMC article. Review.
-
Defensive Traits during White Spruce (Picea glauca) Leaf Ontogeny.Insects. 2021 Jul 15;12(7):644. doi: 10.3390/insects12070644. Insects. 2021. PMID: 34357304 Free PMC article.
-
Hydroxyacetophenone defenses in white spruce against spruce budworm.Evol Appl. 2019 Dec 20;13(1):62-75. doi: 10.1111/eva.12885. eCollection 2020 Jan. Evol Appl. 2019. PMID: 31892944 Free PMC article.
References
-
- Pentzold S, Zagrobelny M, Rook F, Bak S. How insects overcome two-component plant chemical defence: plant β-glucosidases as the main target for herbivore adaptation. Biological Reviews. 2014;89: 531–551. - PubMed
-
- Heckel DG. Insect detoxification and sequestration strategies. Annual Plant Reviews. 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

