Role of efavirenz plasma concentrations on long-term HIV suppression and immune restoration in HIV-infected children
- PMID: 31095608
- PMCID: PMC6521995
- DOI: 10.1371/journal.pone.0216868
Role of efavirenz plasma concentrations on long-term HIV suppression and immune restoration in HIV-infected children
Abstract
Background: To access the long term relationship between efavirenz plasma concentrations and evolution of HIV RNA loads and CD4 cell counts in children.
Methods: Retrospective analysis of data from HIV-infected children on first line efavirenz-containing regimen. A population pharmacokinetic-pharmacodynamic (PK-PD) model was developed to describe the evolution of HIV RNA load and CD4 cell count (efficacy outcomes) in relation to efavirenz plasma concentration. Individual CYP2B6 516 G>T genotype data were not available for this analysis. A score (ISEFV) quantifying the effect of efavirenz concentrations on the long-term HIV replication was calculated from efavirenz concentrations and PD parameters and, a value of ISEFV below which HIV replication is likely not suppressed was determined. Cox proportional hazards regression models were used to assess the association of the risk of viral replication with ISEFV, and with efavirenz mid-dose concentration(C12).
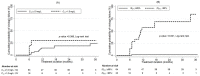
Results: At treatment initiation, median (interquartile range, IQR) age was 8 years (5 to 10), body weight 17 kg (14 to 23), HIV RNA load 5.1 log10 copies/mL (4.6 to 5.4), and CD4 cell count 71 cells/mm3. A model of PK-PD viral dynamics assuming that efavirenz decreases the rate of infected host cells adequately described the relationship of interest. After adjusting for age, baseline HIV RNA load and CD4 cell counts an ISEFV <85% was significantly associated with a higher risk of viral replication (p-value <0.001) while no significant association was observed with C12 <1.0 mg/L.
Conclusion: The ISEFV score was a good predictor of viral replication in children on efavirenz-based treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 30 June 2013. Available from: https://www.who.int/hiv/pub/guidelines/arv2013/download/en/. - PubMed
-
- Emmett SD, Cunningham CK, Mmbaga BT, Kinabo GD, Schimana W, Swai ME, et al. Predicting virologic failure among HIV-1-infected children receiving antiretroviral therapy in Tanzania: a cross-sectional study. J Acquir Immune Defic Syndr. 2010;54(4):368–75. 10.1097/QAI.0b013e3181cf4882 - DOI - PMC - PubMed
-
- Jittamala P, Puthanakit T, Chaiinseeard S, Sirisanthana V. Predictors of virologic failure and genotypic resistance mutation patterns in thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. The Pediatric infectious disease journal. 2009;28(9):826–30. 10.1097/INF.0b013e3181a458f9 - DOI - PubMed
-
- Puthanakit T, Aurpibul L, Oberdorfer P, Akarathum N, Kanjanavanit S, Wannarit P, et al. Sustained immunologic and virologic efficacy after four years of highly active antiretroviral therapy in human immunodeficiency virus infected children in Thailand. The Pediatric infectious disease journal. 2007;26(10):953–6. 10.1097/INF.0b013e318125720a - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

