Berunda Polypeptides: Biheaded Rapamycin Carriers for Subcutaneous Treatment of Autoimmune Dry Eye Disease
- PMID: 31095909
- PMCID: PMC6957305
- DOI: 10.1021/acs.molpharmaceut.9b00263
Berunda Polypeptides: Biheaded Rapamycin Carriers for Subcutaneous Treatment of Autoimmune Dry Eye Disease
Abstract
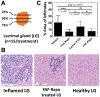
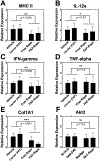
The USFDA-approved immunosuppressive drug rapamycin (Rapa), despite its potency, is limited by poor bioavailability and a narrow therapeutic index. In this study, we sought to improve bioavailability of Rapa with subcutaneous (SC) administration and to test its therapeutic feasibility and practicality in a murine model of Sjögren's syndrome (SS), a systemic autoimmune disease with no approved therapies. To improve its therapeutic index, we formulated Rapa with a carrier termed FAF, a fusion of the human cytosolic FK506-binding protein 12 (FKBP12) and an elastin-like polypeptide (ELP). The resulting 97 kDa FAF (i) has minimal burst release, (ii) is "humanized", (iii) is biodegradable, (iv) solubilizes two Rapa per FAF, and (v) avoids organic solvents or amphiphilic carriers. Demonstrating high stability, FAF remained soluble and monodisperse with a hydrodynamic radius of 8 nm at physiological temperature. A complete pharmacokinetic (PK) analysis of FAF revealed that the bioavailability of SC FAF was 60%, with significantly higher blood concentration during the elimination phase compared to IV FAF. The plasma concentration of Rapa delivered by FAF was 8-fold higher with a significantly increased plasma-to-whole blood ratio relative to free Rapa, 24 h after injection. To evaluate therapeutic effects, FAF-Rapa was administered SC every other day for 2 weeks to male non-obese diabetic (NOD) mice, which develop an SS-like autoimmune-mediated lacrimal gland (LG) inflammation and other characteristic features of SS. Both FAF-Rapa and free Rapa exhibited immunomodulatory effects by significantly suppressing lymphocytic infiltration, gene expression of IFN-γ, MHC II, type I collagen and IL-12a, and cathepsin S (CTSS) activity in LG compared to controls. Serum chemistry and histopathological analyses in major organs revealed no apparent toxicity of FAF-Rapa. Given its improved PK and equipotent therapeutic efficacy compared to free Rapa, FAF-Rapa is of further interest for systemic treatments for autoimmune diseases like SS.
Keywords: FK506-binding protein; Sjögren’s syndrome; cathepsin S; dacryoadenitis; elastin-like polypeptides; lacrimal gland; non-obese diabetic mouse; rapamycin.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.A.M., S.F.H.-A., and S.P. are inventors on patents describing delivery of small molecules using protein polymer fusions related to this work. All other authors declare no competing financial interests.
Figures
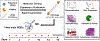

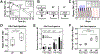
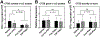

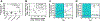
References
-
- Kojima I; Cheng YR; Mohan V; Demain AL Carbon source nutrition of rapamycin biosynthesis in Streptomyces hygroscopicus. J. Ind. Microbiol 1995, 14 (6), 436–9. - PubMed
-
- Costa MA; Simon DI Molecular basis of restenosis and drug-eluting stents. Circulation 2005, 111 (17), 2257–2273. - PubMed
-
- Bjornsti MA; Houghton PJ The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer 2004, 4 (5), 335–48. - PubMed
-
- Hardinger KL; Koch MJ; Brennan DC Current and future immunosuppressive strategies in renal transplantation. Pharmacotherapy 2004, 24 (9), 1159–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

