Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: The PROP UP study
- PMID: 31096006
- PMCID: PMC6959970
- DOI: 10.1016/j.jhep.2019.04.016
Patient-reported symptoms during and after direct-acting antiviral therapies for chronic hepatitis C: The PROP UP study
Abstract
Background & aims: A comprehensive analysis of changes in symptoms and functioning during and after direct-acting antiviral (DAA) therapy for chronic hepatitis C virus (HCV) infection has not been conducted for patients treated in real-world clinical settings. Therefore, we evaluated patient-reported outcomes (PROs) in a diverse cohort of patients with HCV treated with commonly prescribed DAAs.
Methods: PROP UP is a US multicenter observational study of 1,601 patients with HCV treated with DAAs in 2016-2017. PRO data were collected at baseline (T1), early on-treatment (T2), late on-treatment (T3) and 3-months post-treatment (T4). PRO mean change scores were calculated from baseline and a minimally important change (MIC) threshold was set at 5%. Regression analyses investigated patient and treatment characteristics independently associated with PRO changes on-treatment and post-treatment.
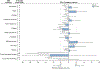
Results: Of 1,564 patients, 55% were male, 39% non-white, 47% had cirrhosis. Sofosbuvir/ledipasvir was prescribed to 63%, sofosbuvir/velpatasvir to 21%, grazoprevir/elbasvir to 11%, and paritaprevir/ombitasvir/ritonavir + dasabuvir to 5%. During DAA therapy, mean PRO scores improved slightly in the overall cohort, but did not reach the 5% MIC threshold. Between 21-53% of patients experienced >5% improved PROs while 23-36% experienced >5% worse symptoms. Of 1,410 patients with evaluable sustained virologic response (SVR) data, 95% achieved SVR. Among those with SVR, all mean PRO scores improved, with the 5% MIC threshold met for fatigue, sleep disturbance, and functioning well-being. Regression analyses identified subgroups, defined by age 35-55, baseline mental health issues and a higher number of health comorbidities as predictors of PRO improvements.
Conclusions: In real-world clinical practices, we observed heterogeneous patient experiences during and after DAA treatment. Symptom improvements were more pronounced in younger patients, those with baseline mental health issues and multiple comorbidities.
Lay summary: Patients who received direct-acting antiviral medications for hepatitis C at several liver centers in the US did not generally experience significant changes in baseline symptoms during treatment. We observed a full range of patient experiences with some patients experiencing substantial symptom improvements, yet others experiencing less improvements and some even experiencing a worsening of symptoms. The 1,346 patients who were cured of hepatitis C experienced improvements in fatigue, sleep disturbance, and functional well-being, and trends for improved pain and depression; whereas the 64 who were not cured experienced minimal improvements. Clinicaltrial.gov: NCT02601820.
Keywords: Functioning; HCV; Liver; PRO; Pain; Patient-reported outcome; Quality of life; Sleep; Symptom; Treatment.
Copyright © 2019 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Reply to: "Patient-reported symptoms during direct-acting antiviral treatment: A real-life study in HIV-HCV coinfected patients (ANRS CO13 HEPAVIH)".J Hepatol. 2020 Mar;72(3):592-593. doi: 10.1016/j.jhep.2019.11.015. Epub 2019 Dec 27. J Hepatol. 2020. PMID: 31889556 No abstract available.
-
Patient-reported symptoms during direct-acting antiviral treatment: A real-life study in HIV-HCV coinfected patients (ANRS CO13 HEPAVIH).J Hepatol. 2020 Mar;72(3):588-591. doi: 10.1016/j.jhep.2019.10.019. Epub 2020 Jan 7. J Hepatol. 2020. PMID: 31924411 No abstract available.
References
-
- Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, Macdonald GA. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J.Pain Symptom.Manage. 2006;31:335–344. - PubMed
-
- Ferri C, Ramos-Casals M, Zignego AL, Arcaini L, Roccatello D, Antonelli A, Saadoun D, et al. International diagnostic guidelines for patients with HCV-related extrahepatic manifestations. A multidisciplinary expert statement. Autoimmun Rev 2016;15:1145–1160. - PubMed
-
- Younossi ZM, Stepanova M, Zeuzem S, Dusheiko G, Esteban R, Hezode C, Reesink HW, et al. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: The VALENCE study. J Hepatol 2014;61:228–234. - PubMed
-
- Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C - the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther 2015;41:497–520. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

