The Relationship of Varenicline Agonism of α4β2 Nicotinic Acetylcholine Receptors and Nicotine-Induced Dopamine Release in Nicotine-Dependent Humans
- PMID: 31096265
- PMCID: PMC7529151
- DOI: 10.1093/ntr/ntz080
The Relationship of Varenicline Agonism of α4β2 Nicotinic Acetylcholine Receptors and Nicotine-Induced Dopamine Release in Nicotine-Dependent Humans
Abstract
Introduction: Cigarette smoking continues to be one of the most important behavioral causes of morbidity and mortality in the world. Varenicline, an α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist, has been shown to increase smoking quit rates compared with nicotine-based products. This human laboratory, double-blind, placebo-controlled study examined varenicline and placebo effects on α4β2-nAChRs occupancy, nicotine-induced change in [11C]raclopride non-displaceable binding potential (BPND), and behavioral measures of cigarette smoking, nicotine craving, and withdrawal.
Methods: Current nicotine dependent daily smokers (N = 17) were randomized to varenicline 1 mg twice daily or placebo for 13 days. Using positron emission tomography), we characterized α4β2-nAChRs occupancy using [18F]AZAN and dopamine receptor binding using [11C]raclopride as well as behavioral measures of cigarettes smoked, craving, and nicotine withdrawal.
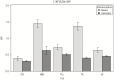
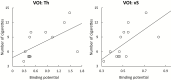
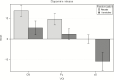
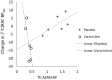
Results: Varenicline compared with placebo resulted in significant reductions in [18F]AZAN BPND in multiple brain regions including thalamus, midbrain, putamen, and ventral striatum. Following administration of a controlled-dose nicotine cigarette, dopamine release was significantly suppressed in the ventral striatum in the varenicline-treated compared with the placebo group. There was a significant relationship between α4β2-nAChRs BPND measured in thalamus during the [18F]AZAN scan and nicotine-induced change in raclopride BPND in the ventral striatum.
Conclusion: This is the first human study to demonstrate a direct relationship between the extent of varenicline occupancy of α4β2-nAChRs and the magnitude of dopamine release following nicotine use.
Implications: It has remained unclear how nicotinic receptor blockade through partial agonist medications such as varenicline promotes smoking cessation. One hypothesized mechanism is downstream dampening of the mesolimbic reward dopamine system. For the first time in human smokers, we observed a direct relationship between the extent of varenicline blockade of α4β2-nACh nicotinic receptors and the magnitude of dopamine release following smoking. This has mechanistic and therapeutic implications for improving smoking cessation interventions.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
The contribution of agonist and antagonist activities of α4β2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data.Psychopharmacology (Berl). 2018 Sep;235(9):2479-2505. doi: 10.1007/s00213-018-4921-9. Epub 2018 Jul 7. Psychopharmacology (Berl). 2018. PMID: 29980822 Review.
-
Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial.JAMA. 2006 Jul 5;296(1):47-55. doi: 10.1001/jama.296.1.47. JAMA. 2006. PMID: 16820546 Clinical Trial.
-
Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid.Neuropharmacology. 2007 Mar;52(3):985-94. doi: 10.1016/j.neuropharm.2006.10.016. Epub 2006 Dec 8. Neuropharmacology. 2007. PMID: 17157884
-
Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers.Clin Ther. 2007 Jun;29(6):1040-56. doi: 10.1016/j.clinthera.2007.06.012. Clin Ther. 2007. PMID: 17692720 Clinical Trial.
-
Varenicline: a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist approved for smoking cessation.Cardiol Rev. 2007 May-Jun;15(3):154-61. doi: 10.1097/01.crd.0000260270.12829.45. Cardiol Rev. 2007. PMID: 17438382 Review.
Cited by
-
Tobacco Smoking and Liver Cancer Risk: Potential Avenues for Carcinogenesis.J Oncol. 2021 Dec 10;2021:5905357. doi: 10.1155/2021/5905357. eCollection 2021. J Oncol. 2021. PMID: 34925509 Free PMC article. Review.
-
Cholinergic tone abnormalities and relationships with smoking severity in human cigarette smokers: exploratory positron emission tomography study using [18F]VAT.Mol Psychiatry. 2025 Sep;30(9):4064-4071. doi: 10.1038/s41380-025-02985-3. Epub 2025 Mar 31. Mol Psychiatry. 2025. PMID: 40164693
-
Imaging Cholinergic Receptors in the Brain by Positron Emission Tomography.J Med Chem. 2023 Aug 24;66(16):10889-10916. doi: 10.1021/acs.jmedchem.3c00573. Epub 2023 Aug 15. J Med Chem. 2023. PMID: 37583063 Free PMC article. Review.
-
An inhibitory brainstem input to dopamine neurons encodes nicotine aversion.Neuron. 2022 Sep 21;110(18):3018-3035.e7. doi: 10.1016/j.neuron.2022.07.003. Epub 2022 Aug 2. Neuron. 2022. PMID: 35921846 Free PMC article.
-
An overview of traditional smoking cessation interventions and E-cigarettes.Front Pharmacol. 2024 Jul 22;15:1293062. doi: 10.3389/fphar.2024.1293062. eCollection 2024. Front Pharmacol. 2024. PMID: 39104396 Free PMC article. Review.
References
-
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl). 1995;117(1):2–10; discussion 14. - PubMed
-
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390(6658):401–404. - PubMed
-
- Gonzales D, Rennard SI, Nides M, et al. ; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

