Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells
- PMID: 31096595
- PMCID: PMC6566394
- DOI: 10.3390/nu11051077
Polyphenol-Enriched Plum Extract Enhances Myotubule Formation and Anabolism while Attenuating Colon Cancer-induced Cellular Damage in C2C12 Cells
Abstract
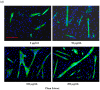
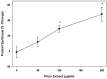
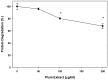
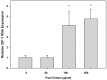
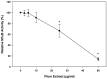
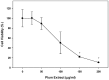
Preventing muscle wasting in certain chronic diseases including cancer is an ongoing challenge. Studies have shown that polyphenols derived from fruits and vegetables shows promise in reducing muscle loss in cellular and animal models of muscle wasting. We hypothesized that polyphenols derived from plums (Prunus domestica) could have anabolic and anti-catabolic benefits on skeletal muscle. The effects of a polyphenol-enriched plum extract (PE60) were evaluated in vitro on C2C12 and Colon-26 cancer cells. Data were analyzed using a one-way ANOVA and we found that treatment of myocytes with plum extract increased the cell size by ~3-fold (p < 0.05) and stimulated myoblast differentiation by ~2-fold (p < 0.05). Plum extract induced total protein synthesis by ~50% (p < 0.05), reduced serum deprivation-induced total protein degradation by ~30% (p < 0.05), and increased expression of Insulin-Like Growth Factor-1 (IGF-1) by ~2-fold (p < 0.05). Plum extract also reduced tumor necrosis factor α (TNFα)-induced nuclear factor κB (NFκB) activation by 80% (p < 0.05) in A549/NF-κB-luc cells. In addition, plum extract inhibited the growth of Colon-26 cancer cells, and attenuated cytotoxicity in C2C12 myoblasts induced by soluble factors released from Colon-26 cells. In conclusion, our data suggests that plum extract may have pluripotent health benefits on muscle, due to its demonstrated ability to promote myogenesis, stimulate muscle protein synthesis, and inhibit protein degradation. It also appears to protect muscle cell from tumor-induced cytotoxicity.
Keywords: cachexia; cancer; muscle wasting; myoblasts; plum; protein synthesis.
Conflict of interest statement
Suzette Pereira and Paul Johns are currently employed by Abbott. Padmavathy Krishnan is an ex-Abbott employee. Rafat Siddiqui, Haiwen Li, and Faten Alsolmei have no conflict of interest.
Figures





References
-
- Warren S. The immediate causes of death in cancer. Am. J. Med. Sci. 1932;184:610–616. doi: 10.1097/00000441-193211000-00002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

