Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones
- PMID: 31096601
- PMCID: PMC6572547
- DOI: 10.3390/molecules24101868
Synthesis and Cytotoxic Evaluation of 3-Methylidenechroman-4-ones
Abstract
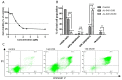
In the search for new anticancer agents, a library of variously substituted 3-methylidenechroman-4-ones was synthesized using Horner-Wadsworth-Emmons methodology. Acylation of diethyl methylphosphonate with selected ethyl salicylates furnished 3-diethoxyphosphorylchromen-4-ones which were next used as Michael acceptors in the reaction with various Grignard reagents. The adducts were obtained as the mixtures of trans and cis diastereoisomers along with a small amount of enol forms. Their relative configuration and preferred conformation were established by NMR analysis. The adducts turned up to be effective Horner-Wadsworth-Emmons reagents giving 2-substituted 3-methylidenechroman-4-ones, which were then tested for their possible cytotoxic activity against two leukemia cell lines, HL-60 and NALM-6, and against MCF-7 breast cancer cell line. All new compounds (14a-o) were highly cytotoxic for the leukemic cells and showed a moderate or weak effect on MCF-7 cells. Analog 14d exhibited the highest growth inhibitory activity and was more potent than carboplatin against HL-60 (IC50 = 1.46 ± 0.16 µM) and NALM-6 (IC50 = 0.50 ± 0.05 µM) cells. Further tests showed that 14d induced apoptosis in NALM-6 cells, which was mediated mostly through the extrinsic pathway.
Keywords: 3-methylidenechroman-4-ones; Horner–Wadsworth–Emmons olefination; Michael addition; apoptosis; cancer cell lines.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


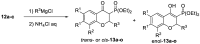
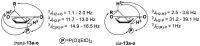



References
-
- Huang S., Zhao Y., Zhou X., Wu Y., Wu P., Liu T., Yang B., Hu Y., Dong X. Design, synthesis and biological evaluation of 3-benzylideneflavanone derivatives as cytotoxic agents. Med. Chem. Res. 2012;21:4150–4157. doi: 10.1007/s00044-011-9959-8. - DOI
-
- Sidwell W.T.L., Tamm C. The homo-isoflavones II. Isolation and structure of 4′-O-methyl-punctatin, autumnalin and 3, 9-dihydro-autumnalin. Tetrahedron Lett. 1970:475–478. doi: 10.1016/0040-4039(70)89003-7. - DOI
-
- Böhler P., Tamm C. The homo-isoflavones, a new class of natural product. Isolation and structure of eucomin and eucomol. Tetrahedron Lett. 1967;8:3479–3483. doi: 10.1016/S0040-4039(01)89826-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

