Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases
- PMID: 31096608
- PMCID: PMC6566277
- DOI: 10.3390/ijms20102407
Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases
Abstract
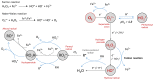
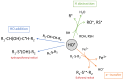
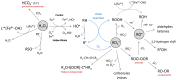
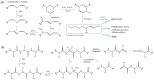
Increasing numbers of individuals suffer from neurodegenerative diseases, which are characterized by progressive loss of neurons. Oxidative stress, in particular, the overproduction of Reactive Oxygen Species (ROS), play an important role in the development of these diseases, as evidenced by the detection of products of lipid, protein and DNA oxidation in vivo. Even if they participate in cell signaling and metabolism regulation, ROS are also formidable weapons against most of the biological materials because of their intrinsic nature. By nature too, neurons are particularly sensitive to oxidation because of their high polyunsaturated fatty acid content, weak antioxidant defense and high oxygen consumption. Thus, the overproduction of ROS in neurons appears as particularly deleterious and the mechanisms involved in oxidative degradation of biomolecules are numerous and complexes. This review highlights the production and regulation of ROS, their chemical properties, both from kinetic and thermodynamic points of view, the links between them, and their implication in neurodegenerative diseases.
Keywords: NADPH oxidase; hydrogen peroxide; hydroperoxides; hydroxyl radical; neurodegenerative diseases; reactive oxygen species; superoxide anion; superoxide dismutase.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 3rd ed. Oxford science Publications; Oxford, UK: 1999.
-
- Neta P., Huie R.E., Ross A.B. Rate constants for reactions of peroxyl radicals in fluid solutions. J. Phys. Chem. Ref. Data. 1990;19:413–513. doi: 10.1063/1.555854. - DOI
-
- Haber F., Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. 1934;147:332–351.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

