Aedes aegypti saliva impairs M1-associated proinflammatory phenotype without promoting or affecting M2 polarization of murine macrophages
- PMID: 31097013
- PMCID: PMC6524299
- DOI: 10.1186/s13071-019-3487-7
Aedes aegypti saliva impairs M1-associated proinflammatory phenotype without promoting or affecting M2 polarization of murine macrophages
Abstract
Background: During the feeding process, the mouthparts of hematophagous mosquitoes break the skin barrier and probe the host tissue to find the blood. The saliva inoculated in this microenvironment modulates host hemostasis, inflammation and adaptive immune responses. However, the mechanisms involved in these biological activities remain poorly understood and few studies explored the potential roles of mosquito saliva on the individual cellular components of the immune system. Here, we report the immunomodulatory activities of Aedes aegypti salivary cocktail on murine peritoneal macrophages.
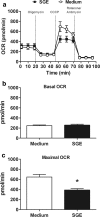
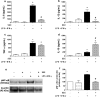
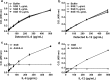
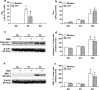
Results: The salivary gland extract (SGE) of Ae. aegypti inhibited the production of nitric oxide and inflammatory cytokines such as interleukin-6 (IL-6) and IL-12, as well as the expression of inducible nitric oxide synthase and NF-κB by murine macrophages stimulated by lipopolysaccharide (LPS) plus interferon-γ (IFN-γ). The spare respiratory capacity, the phagocytic and microbicidal activities of these macrophages were also reduced by Ae. aegypti SGE. These phenotypic changes are consistent with SGE suppressing the proinflammatory program of M1 macrophages. On the other hand, Ae. aegypti SGE did not influence M2-associated markers (urea production, arginase-1 and mannose receptor-1 expression), either in macrophages alternatively activated by IL-4 or in those classically activated by LPS plus IFN-γ. In addition, Ae. aegypti SGE did not display any cytokine-binding activity, nor did it affect macrophage viability, thus excluding supposed experimental artifacts.
Conclusions: Given the importance of macrophages in a number of biological processes, our findings help to enlighten how vector saliva modulates vertebrate host immunity.
Keywords: Aedes aegypti; Inflammation; M1/M2 polarization; Macrophages; Saliva; microbicidal activity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
AeMOPE-1, a Novel Salivary Peptide From Aedes aegypti, Selectively Modulates Activation of Murine Macrophages and Ameliorates Experimental Colitis.Front Immunol. 2021 Jul 19;12:681671. doi: 10.3389/fimmu.2021.681671. eCollection 2021. Front Immunol. 2021. PMID: 34349757 Free PMC article.
-
Saliva of the Yellow Fever mosquito, Aedes aegypti, modulates murine lymphocyte function.Parasite Immunol. 2004 Jun-Jul;26(6-7):295-306. doi: 10.1111/j.0141-9838.2004.00712.x. Parasite Immunol. 2004. PMID: 15541033
-
Specific human antibody responses to Aedes aegypti and Aedes polynesiensis saliva: A new epidemiological tool to assess human exposure to disease vectors in the Pacific.PLoS Negl Trop Dis. 2018 Jul 24;12(7):e0006660. doi: 10.1371/journal.pntd.0006660. eCollection 2018 Jul. PLoS Negl Trop Dis. 2018. PMID: 30040826 Free PMC article.
-
Aedes aegypti salivary gland extract ameliorates experimental inflammatory bowel disease.Int Immunopharmacol. 2015 May;26(1):13-22. doi: 10.1016/j.intimp.2015.03.002. Epub 2015 Mar 12. Int Immunopharmacol. 2015. PMID: 25770821
-
Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages.Front Immunol. 2019 May 24;10:1084. doi: 10.3389/fimmu.2019.01084. eCollection 2019. Front Immunol. 2019. PMID: 31178859 Free PMC article. Review.
Cited by
-
Aedes aegypti mosquito saliva ameliorates acetaminophen-induced liver injury in mice.PLoS One. 2021 Feb 8;16(2):e0245788. doi: 10.1371/journal.pone.0245788. eCollection 2021. PLoS One. 2021. PMID: 33556084 Free PMC article.
-
AeMOPE-1, a Novel Salivary Peptide From Aedes aegypti, Selectively Modulates Activation of Murine Macrophages and Ameliorates Experimental Colitis.Front Immunol. 2021 Jul 19;12:681671. doi: 10.3389/fimmu.2021.681671. eCollection 2021. Front Immunol. 2021. PMID: 34349757 Free PMC article.
-
Evaluation of inflammatory skin infiltrate following Aedes aegypti bites in sensitized and non-sensitized mice reveals saliva-dependent and immune-dependent phenotypes.Immunology. 2019 Sep;158(1):47-59. doi: 10.1111/imm.13096. Immunology. 2019. PMID: 31315156 Free PMC article.
-
Aedes aegypti salivary gland extract alleviates acute itching by blocking TRPA1 channels.Front Physiol. 2023 Jun 27;14:1055706. doi: 10.3389/fphys.2023.1055706. eCollection 2023. Front Physiol. 2023. PMID: 37441000 Free PMC article.
-
Influence of Host-Related Factors and Exposure to Mosquito Bites on the Dynamics of Antibody Response to Plasmodium falciparum Antigens.Trop Med Infect Dis. 2021 Oct 18;6(4):185. doi: 10.3390/tropicalmed6040185. Trop Med Infect Dis. 2021. PMID: 34698307 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2015/22934-0/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2016/04921-1/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2017/11540-7/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 12.1.17661.1.7/Núcleo de Pesquisa em Moléculas Bioativas de Artrópoes Vetores
- 465678/2014-9/Conselho Nacional de Desenvolvimento Científico e Tecnológico
LinkOut - more resources
Full Text Sources
Research Materials

