The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes
- PMID: 31097328
- PMCID: PMC6589397
- DOI: 10.1016/j.kint.2019.02.014
The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes
Abstract
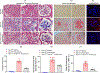
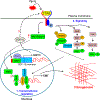
Transforming growth factor-β (TGF-β) is known to play a critical role in the pathogenesis of many progressive podocyte diseases. However, the molecular mechanisms regulating TGF-β signaling in podocytes remain unclear. Using a podocyte-specific myosin (Myo)1c knockout, we demonstrate whether Myo1c is critical for TGF-β-signaling in podocyte disease pathogenesis. Specifically, podocyte-specific Myo1c knockout mice were resistant to fibrotic injury induced by Adriamycin or nephrotoxic serum. Further, loss of Myo1c also protected from injury in the TGF-β-dependent unilateral ureteral obstruction mouse model of renal interstitial fibrosis. Mechanistic analyses showed that loss of Myo1c significantly blunted TGF-β signaling through downregulation of canonical and non-canonical TGF-β pathways. Interestingly, nuclear rather than the cytoplasmic Myo1c was found to play a central role in controlling TGF-β signaling through transcriptional regulation. Differential expression analysis of nuclear Myo1c-associated gene promoters showed that nuclear Myo1c targeted the TGF-β responsive gene growth differentiation factor (GDF)-15 and directly bound to the GDF-15 promoter. Importantly, GDF15 was found to be involved in podocyte pathogenesis, where GDF15 was upregulated in glomeruli of patients with focal segmental glomerulosclerosis. Thus, Myo1c-mediated regulation of TGF-β-responsive genes is central to the pathogenesis of podocyte injury. Hence, inhibiting this process may have clinical application in treating podocytopathies.
Keywords: TGF-beta; fibrosis; focal segmental glomerulosclerosis; glomerulonephritis; glomerulus; podocyte.
Copyright © 2019 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures







References
-
- Mundel P and Shankland SJ, Podocyte biology and response to injury. J Am Soc Nephrol. 13:3005–3015, 2002 - PubMed
-
- Shankland SJ, The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69:2131–2147, 2006 - PubMed
-
- Cellesi F, Li M, and Rastaldi MP, Podocyte injury and repair mechanisms. Curr Opin Nephrol Hypertens. 24:239–244, 2015 - PubMed
-
- Nagata M, Podocyte injury and its consequences. Kidney Int. 89:1221–1230, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

