Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes
- PMID: 31097468
- PMCID: PMC6605759
- DOI: 10.1084/jem.20182386
Sex-specific effects of microbiome perturbations on cerebral Aβ amyloidosis and microglia phenotypes
Abstract
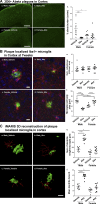
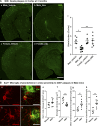
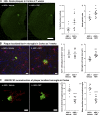
We demonstrated that an antibiotic cocktail (ABX)-perturbed gut microbiome is associated with reduced amyloid-β (Aβ) plaque pathology and astrogliosis in the male amyloid precursor protein (APP)SWE /presenilin 1 (PS1)ΔE9 transgenic model of Aβ amyloidosis. We now show that in an independent, aggressive APPSWE/PS1L166P (APPPS1-21) mouse model of Aβ amyloidosis, an ABX-perturbed gut microbiome is associated with a reduction in Aβ pathology and alterations in microglial morphology, thus establishing the generality of the phenomenon. Most importantly, these latter alterations occur only in brains of male mice, not in the brains of female mice. Furthermore, ABX treatment lead to alterations in levels of selected microglial expressed transcripts indicative of the "M0" homeostatic state in male but not in female mice. Finally, we found that transplants of fecal microbiota from age-matched APPPS1-21 male mice into ABX-treated APPPS1-21 male restores the gut microbiome and partially restores Aβ pathology and microglial morphology, thus demonstrating a causal role of the microbiome in the modulation of Aβ amyloidosis and microglial physiology in mouse models of Aβ amyloidosis.
© 2019 Dodiya et al.
Figures



References
-
- Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z., Liu C., Klotz L., Stauffer U., Baranzini S.E., et al. . 2017. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA. 114:10719–10724. 10.1073/pnas.1711233114 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

