Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species
- PMID: 31097580
- PMCID: PMC6561167
- DOI: 10.1073/pnas.1901777116
Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species
Erratum in
-
Correction for Montay-Gruel et al., Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species.Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25946-25947. doi: 10.1073/pnas.2019057117. Epub 2020 Oct 5. Proc Natl Acad Sci U S A. 2020. PMID: 33020294 Free PMC article. No abstract available.
Abstract
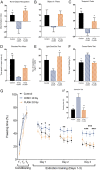
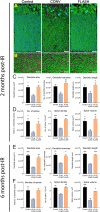
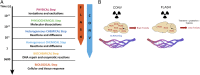
Here, we highlight the potential translational benefits of delivering FLASH radiotherapy using ultra-high dose rates (>100 Gy⋅s-1). Compared with conventional dose-rate (CONV; 0.07-0.1 Gy⋅s-1) modalities, we showed that FLASH did not cause radiation-induced deficits in learning and memory in mice. Moreover, 6 months after exposure, CONV caused permanent alterations in neurocognitive end points, whereas FLASH did not induce behaviors characteristic of anxiety and depression and did not impair extinction memory. Mechanistic investigations showed that increasing the oxygen tension in the brain through carbogen breathing reversed the neuroprotective effects of FLASH, while radiochemical studies confirmed that FLASH produced lower levels of the toxic reactive oxygen species hydrogen peroxide. In addition, FLASH did not induce neuroinflammation, a process described as oxidative stress-dependent, and was also associated with a marked preservation of neuronal morphology and dendritic spine density. The remarkable normal tissue sparing afforded by FLASH may someday provide heretofore unrealized opportunities for dose escalation to the tumor bed, capabilities that promise to hasten the translation of this groundbreaking irradiation modality into clinical practice.
Keywords: cognitive dysfunction; neuroinflammation; neuronal morphology; reactive oxygen species; ultra-high dose-rate irradiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Begg AC, Stewart FA, Vens C (2011) Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 11:239–253. - PubMed
-
- Bentzen SM. (2006) Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat Rev Cancer 6:702–713. - PubMed
-
- Yarnold J, Brotons MC (2010) Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 97:149–161. - PubMed
-
- Bortfeld T. (2006) IMRT: A review and preview. Phys Med Biol 51:R363–R379. - PubMed
-
- Butler JM, Rapp SR, Shaw EG (2006) Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol 7:517–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

