Increased Lysosomal Exocytosis Induced by Lysosomal Ca2+ Channel Agonists Protects Human Dopaminergic Neurons from α-Synuclein Toxicity
- PMID: 31097622
- PMCID: PMC6636071
- DOI: 10.1523/JNEUROSCI.3085-18.2019
Increased Lysosomal Exocytosis Induced by Lysosomal Ca2+ Channel Agonists Protects Human Dopaminergic Neurons from α-Synuclein Toxicity
Abstract
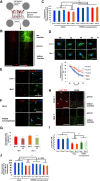
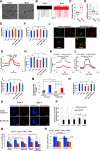
The accumulation of misfolded proteins is a common pathological feature of many neurodegenerative disorders, including synucleinopathies such as Parkinson's disease (PD), which is characterized by the presence of α-synuclein (α-syn)-containing Lewy bodies. However, although recent studies have investigated α-syn accumulation and propagation in neurons, the molecular mechanisms underlying α-syn transmission have been largely unexplored. Here, we examined a monogenic form of synucleinopathy caused by loss-of-function mutations in lysosomal ATP13A2/PARK9. These studies revealed that lysosomal exocytosis regulates intracellular levels of α-syn in human neurons. Loss of PARK9 function in patient-derived dopaminergic neurons disrupted lysosomal Ca2+ homeostasis, reduced lysosomal Ca2+ storage, increased cytosolic Ca2+, and impaired lysosomal exocytosis. Importantly, this dysfunction in lysosomal exocytosis impaired α-syn secretion from both axons and soma, promoting α-syn accumulation. However, activation of the lysosomal Ca2+ channel transient receptor potential mucolipin 1 (TRPML1) was sufficient to upregulate lysosomal exocytosis, rescue defective α-syn secretion, and prevent α-syn accumulation. Together, these results suggest that intracellular α-syn levels are regulated by lysosomal exocytosis in human dopaminergic neurons and may represent a potential therapeutic target for PD and other synucleinopathies.SIGNIFICANCE STATEMENT Parkinson's disease (PD) is the second most common neurodegenerative disease linked to the accumulation of α-synuclein (α-syn) in patient neurons. However, it is unclear what the mechanism might be. Here, we demonstrate a novel role for lysosomal exocytosis in clearing intracellular α-syn and show that impairment of this pathway by mutations in the PD-linked gene ATP13A2/PARK9 contributes to α-syn accumulation in human dopaminergic neurons. Importantly, upregulating lysosomal exocytosis by increasing lysosomal Ca2+ levels was sufficient to rescue defective α-syn secretion and accumulation in patient neurons. These studies identify lysosomal exocytosis as a potential therapeutic target in diseases characterized by the accumulation of α-syn, including PD.
Keywords: Kufor–Rakeb syndrome; Parkinson's disease; TRPML1; alpha synuclein; dopaminergic neuron; lysosomal exocytosis.
Copyright © 2019 the authors.
Figures



References
-
- Bliederhaeuser C, Grozdanov V, Speidel A, Zondler L, Ruf WP, Bayer H, Kiechle M, Feiler MS, Freischmidt A, Brenner D, Witting A, Hengerer B, Fändrich M, Ludolph AC, Weishaupt JH, Gillardon F, Danzer KM (2016) Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol 131:379–391. 10.1007/s00401-015-1504-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
