Regulation of Neuronal Survival and Axon Growth by a Perinuclear cAMP Compartment
- PMID: 31097623
- PMCID: PMC6616289
- DOI: 10.1523/JNEUROSCI.2752-18.2019
Regulation of Neuronal Survival and Axon Growth by a Perinuclear cAMP Compartment
Abstract
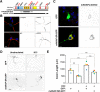
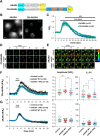
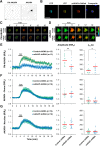
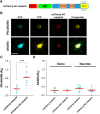
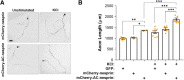
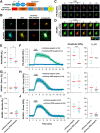
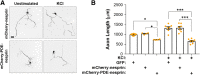
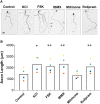
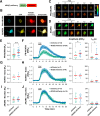
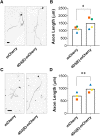
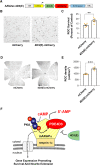
cAMP signaling is known to be critical in neuronal survival and axon growth. Increasingly the subcellular compartmentation of cAMP signaling has been appreciated, but outside of dendritic synaptic regulation, few cAMP compartments have been defined in terms of molecular composition or function in neurons. Specificity in cAMP signaling is conferred in large part by A-kinase anchoring proteins (AKAPs) that localize protein kinase A and other signaling enzymes to discrete intracellular compartments. We now reveal that cAMP signaling within a perinuclear neuronal compartment organized by the large multivalent scaffold protein mAKAPα promotes neuronal survival and axon growth. mAKAPα signalosome function is explored using new molecular tools designed to specifically alter local cAMP levels as studied by live-cell FRET imaging. In addition, enhancement of mAKAPα-associated cAMP signaling by isoform-specific displacement of bound phosphodiesterase is demonstrated to increase retinal ganglion cell survival in vivo in mice of both sexes following optic nerve crush injury. These findings define a novel neuronal compartment that confers cAMP regulation of neuroprotection and axon growth and that may be therapeutically targeted in disease.SIGNIFICANCE STATEMENT cAMP is a second messenger responsible for the regulation of diverse cellular processes including neuronal neurite extension and survival following injury. Signal transduction by cAMP is highly compartmentalized in large part because of the formation of discrete, localized multimolecular signaling complexes by A-kinase anchoring proteins. Although the concept of cAMP compartmentation is well established, the function and identity of these compartments remain poorly understood in neurons. In this study, we provide evidence for a neuronal perinuclear cAMP compartment organized by the scaffold protein mAKAPα that is necessary and sufficient for the induction of neurite outgrowth in vitro and for the survival of retinal ganglion cells in vivo following optic nerve injury.
Keywords: FRET imaging; cAMP; neuroprotection; phosphodiesterase; signal transduction; signalosome.
Copyright © 2019 the authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
