Long-term event-free survival, chimerism and fertility outcomes in 234 patients with sickle-cell anemia younger than 30 years after myeloablative conditioning and matched-sibling transplantation in France
- PMID: 31097628
- PMCID: PMC6939536
- DOI: 10.3324/haematol.2018.213207
Long-term event-free survival, chimerism and fertility outcomes in 234 patients with sickle-cell anemia younger than 30 years after myeloablative conditioning and matched-sibling transplantation in France
Abstract
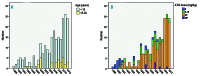
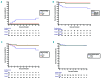
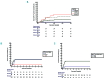
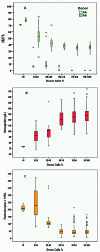
Allogeneic stem cell transplantation remains the only curative treatment for sickle cell anemia (SCA), but the place of myeloablative conditioning in the procedure remains to be defined. The aim of the present study was to analyze long-term outcomes, including chimerism, SCA-related events and biological data (hemoglobin, reticulocytes, HbS%), and fertility in a French series of 234 SCA patients under 30 years of age who, from 1988 to 2012, received a matched-sibling-donor stem cell transplantation following standardized myeloablative conditioning [busulfan, cyclophosphamide and rabbit antithymocyte globulin (ATG)]. Since the first report of the series (1988-2004), 151 new consecutive patients with SCA have been similarly transplanted. Considering death, non-engraftment or rejection (donor cells <5%) as events, the 5-year event-free survival was 97.9% (95% confidence interval: 95.5-100%), confirming, since the year 2000, an at least 95% chance of cure. In the overall cohort (n=234, median follow up 7.9 years), event-free survival was not associated with age, but chronic-graft-versus-host disease (cGvHD) was independently associated with recipient's age >15 years (hazard ratio=4.37; P=0.002) and lower (5-15 vs 20 mg/kg) ATG dose (hazard ratio=4.55; P=0.001). At one year, 44% of patients had mixed chimerism (5-95% donor cells), but those prepared with ATG had no graft rejection. No events related to SCA occurred in patients with mixed chimerism, even those with 15-20% donor cells, but hemolytic anemia stigmata were observed with donor cells <50%. Myeloablative transplantation with matched-sibling donor currently has a higher event-free survival (98%) in patients under 30 years of age than that reported for non-myeloablative conditioning (88%). Nevertheless, the risk of cGvHD in older patients and the need to preserve fertility might be indications for a non-myeloablative conditioning.
Copyright© 2020 Ferrata Storti Foundation.
Figures
References
-
- Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. N Engl J Med. 1986;314(25):1593–1599. - PubMed
-
- Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121(3):562–569. - PubMed
-
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crisis in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. - PubMed
-
- Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88(6):1960–1964. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

