Hydrodealkenylative C(sp3)-C(sp2) bond fragmentation
- PMID: 31097667
- PMCID: PMC6625654
- DOI: 10.1126/science.aaw4212
Hydrodealkenylative C(sp3)-C(sp2) bond fragmentation
Abstract
Chemical synthesis typically relies on reactions that generate complexity through elaboration of simple starting materials. Less common are deconstructive strategies toward complexity-particularly those involving carbon-carbon bond scission. Here, we introduce one such transformation: the hydrodealkenylative cleavage of C(sp3)-C(sp2) bonds, conducted below room temperature, using ozone, an iron salt, and a hydrogen atom donor. These reactions are performed in nonanhydrous solvents and open to the air; reach completion within 30 minutes; and deliver their products in high yields, even on decagram scales. We have used this broadly functionality tolerant transformation to produce desirable synthetic intermediates, many of which are optically active, from abundantly available terpenes and terpenoid-derived precursors. We have also applied it in the formal total syntheses of complex molecules.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

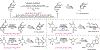
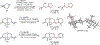
Comment in
-
Curbing the costs of chemical manufacturing.Science. 2019 May 17;364(6441):635. doi: 10.1126/science.aax2613. Science. 2019. PMID: 31097654 No abstract available.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

