Sam50-Mic19-Mic60 axis determines mitochondrial cristae architecture by mediating mitochondrial outer and inner membrane contact
- PMID: 31097788
- PMCID: PMC7206006
- DOI: 10.1038/s41418-019-0345-2
Sam50-Mic19-Mic60 axis determines mitochondrial cristae architecture by mediating mitochondrial outer and inner membrane contact
Abstract
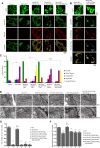
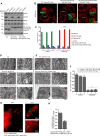
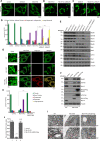
Mitochondrial cristae are critical for efficient oxidative phosphorylation, however, how cristae architecture is precisely organized remains largely unknown. Here, we discovered that Mic19, a core component of MICOS (mitochondrial contact site and cristae organizing system) complex, can be cleaved at N-terminal by mitochondrial protease OMA1 under certain physiological stresses. Mic19 directly interacts with mitochondrial outer-membrane protein Sam50 (the key subunit of SAM complex) and inner-membrane protein Mic60 (the key component of MICOS complex) to form Sam50-Mic19-Mic60 axis, which dominantly connects SAM and MICOS complexes to assemble MIB (mitochondrial intermembrane space bridging) supercomplex for mediating mitochondrial outer- and inner-membrane contact. OMA1-mediated Mic19 cleavage causes Sam50-Mic19-Mic60 axis disruption, which separates SAM and MICOS and leads to MIB disassembly. Disrupted Sam50-Mic19-Mic60 axis, even in the presence of SAM and MICOS complexes, causes the abnormal mitochondrial morphology, loss of mitochondrial cristae junctions, abnormal cristae distribution and reduced ATP production. Importantly, Sam50 displays punctate distribution at mitochondrial outer membrane, and acts as an anchoring point to guide the formation of mitochondrial cristae junctions. Therefore, we propose that Sam50-Mic19-Mic60 axis-mediated SAM-MICOS complexes integration determines mitochondrial cristae architecture.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Sub-mitochondrial localization of the genetic-tagged mitochondrial intermembrane space-bridging components Mic19, Mic60 and Sam50.J Cell Sci. 2017 Oct 1;130(19):3248-3260. doi: 10.1242/jcs.201400. Epub 2017 Aug 14. J Cell Sci. 2017. PMID: 28808085 Free PMC article.
-
Detailed analysis of the human mitochondrial contact site complex indicate a hierarchy of subunits.PLoS One. 2015 Mar 17;10(3):e0120213. doi: 10.1371/journal.pone.0120213. eCollection 2015. PLoS One. 2015. PMID: 25781180 Free PMC article.
-
The Oxidation Status of Mic19 Regulates MICOS Assembly.Mol Cell Biol. 2015 Dec;35(24):4222-37. doi: 10.1128/MCB.00578-15. Epub 2015 Sep 28. Mol Cell Biol. 2015. PMID: 26416881 Free PMC article.
-
Mitochondrial inner membrane protein, Mic60/mitofilin in mammalian organ protection.J Cell Physiol. 2019 Apr;234(4):3383-3393. doi: 10.1002/jcp.27314. Epub 2018 Sep 14. J Cell Physiol. 2019. PMID: 30259514 Free PMC article. Review.
-
Mitochondrial contact site and cristae organizing system.Curr Opin Cell Biol. 2016 Aug;41:33-42. doi: 10.1016/j.ceb.2016.03.013. Epub 2016 Apr 7. Curr Opin Cell Biol. 2016. PMID: 27062547 Review.
Cited by
-
Sam50 exerts neuroprotection by maintaining the mitochondrial structure during experimental cerebral ischemia/reperfusion injury in rats.CNS Neurosci Ther. 2022 Dec;28(12):2230-2244. doi: 10.1111/cns.13967. Epub 2022 Sep 8. CNS Neurosci Ther. 2022. PMID: 36074556 Free PMC article.
-
Molecular machineries shaping the mitochondrial inner membrane.Nat Rev Mol Cell Biol. 2025 Sep;26(9):706-724. doi: 10.1038/s41580-025-00854-z. Epub 2025 May 14. Nat Rev Mol Cell Biol. 2025. PMID: 40369159 Review.
-
TRAP1 inhibits MIC60 ubiquitination to mitigate the injury of cardiomyocytes and protect mitochondria in extracellular acidosis.Cell Death Discov. 2021 Dec 14;7(1):389. doi: 10.1038/s41420-021-00786-5. Cell Death Discov. 2021. PMID: 34907169 Free PMC article.
-
TRAP1 inhibits MARCH5-mediated MIC60 degradation to alleviate mitochondrial dysfunction and apoptosis of cardiomyocytes under diabetic conditions.Cell Death Differ. 2023 Oct;30(10):2336-2350. doi: 10.1038/s41418-023-01218-w. Epub 2023 Sep 7. Cell Death Differ. 2023. PMID: 37679468 Free PMC article.
-
OPA1 and MICOS Regulate mitochondrial crista dynamics and formation.Cell Death Dis. 2020 Oct 31;11(10):940. doi: 10.1038/s41419-020-03152-y. Cell Death Dis. 2020. PMID: 33130824 Free PMC article.
References
-
- Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542–8. - PubMed
-
- Demongeot J, Glade N, Hansen O, Moreira A. An open issue: the inner mitochondrial membrane (IMM) as a free boundary problem. Biochimie. 2007;89:1049–57. - PubMed
-
- Huynen MA, Muhlmeister M, Gotthardt K, Guerrero-Castillo S, Brandt U. Evolution and structural organization of the mitochondrial contact site (MICOS) complex and the mitochondrial intermembrane space bridging (MIB) complex. Biochim Biophys Acta. 2016;1863:91–101. - PubMed
-
- van der Laan M, Horvath SE, Pfanner N. Mitochondrial contact site and cristae organizing system. Curr Opin Cell Biol. 2016;41:33–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

