Controlling hypoxia-inducible factor-2α is critical for maintaining bone homeostasis in mice
- PMID: 31098335
- PMCID: PMC6513851
- DOI: 10.1038/s41413-019-0054-y
Controlling hypoxia-inducible factor-2α is critical for maintaining bone homeostasis in mice
Abstract
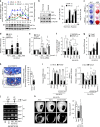
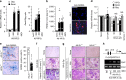
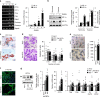
Pathological bone loss is caused by an imbalance between bone formation and resorption. The bone microenvironments are hypoxic, and hypoxia-inducible factor (HIF) is known to play notable roles in bone remodeling. However, the relevant functions of HIF-2α are not well understood. Here, we have shown that HIF-2α deficiency in mice enhances bone mass through its effects on the differentiation of osteoblasts and osteoclasts. In vitro analyses revealed that HIF-2α inhibits osteoblast differentiation by targeting Twist2 and stimulates RANKL-induced osteoclastogenesis via regulation of Traf6. In addition, HIF-2α appears to contribute to the crosstalk between osteoblasts and osteoclasts by directly targeting RANKL in osteoprogenitor cells. Experiments performed with osteoblast- and osteoclast-specific conditional knockout mice supported a role of HIF-2α in this crosstalk. HIF-2α deficiency alleviated ovariectomy-induced bone loss in mice, and specific inhibition of HIF-2α with ZINC04179524 significantly blocked RANKL-mediated osteoclastogenesis. Collectively, our results suggest that HIF-2α functions as a catabolic regulator in bone remodeling, which is critical for the maintenance of bone homeostasis.
Keywords: Bone; Diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

