Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults
- PMID: 31098397
- PMCID: PMC6517691
- DOI: 10.1128/mSystems.00261-19
Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults
Abstract
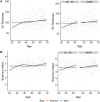
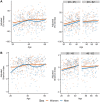
Gut microbial diversity changes throughout the human life span and is known to be associated with host sex. We investigated the association of age, sex, and gut bacterial alpha diversity in three large cohorts of adults from four geographical regions: subjects from the United States and United Kingdom in the American Gut Project (AGP) citizen-science initiative and two independent cohorts of Colombians and Chinese. In three of the four cohorts, we observed a strong positive association between age and alpha diversity in young adults that plateaued after age 40 years. We also found sex-dependent differences that were more pronounced in younger adults than in middle-aged adults, with women having higher alpha diversity than men. In contrast to the other three cohorts, no association of alpha diversity with age or sex was observed in the Chinese cohort. The association of alpha diversity with age and sex remained after adjusting for cardiometabolic parameters in the Colombian cohort and antibiotic usage in the AGP cohort. We further attempted to predict the microbiota age in individuals using a machine-learning approach for the men and women in each cohort. Consistent with our alpha-diversity-based findings, U.S. and U.K. women had a significantly higher predicted microbiota age than men, with a reduced difference being seen above age 40 years. This difference was not observed in the Colombian cohort and was observed only in middle-aged Chinese adults. Together, our results provide new insights into the influence of age and sex on the biodiversity of the human gut microbiota during adulthood while highlighting similarities and differences across diverse cohorts. IMPORTANCE Microorganisms in the human gut play a role in health and disease, and in adults higher gut biodiversity has been linked to better health. Since gut microorganisms may be pivotal in the development of microbial therapies, understanding the factors that shape gut biodiversity is of utmost interest. We performed large-scale analyses of the relationship of age and sex to gut bacterial diversity in adult cohorts from four geographic regions: the United States, the United Kingdom, Colombia, and China. In the U.S., U.K., and Colombian cohorts, bacterial biodiversity correlated positively with age in young adults but plateaued at about age 40 years, with no positive association being found in middle-aged adults. Young, but not middle-aged, adult women had higher gut bacterial diversity than men, a pattern confirmed via supervised machine learning. Interestingly, in the Chinese cohort, minimal associations were observed between gut biodiversity and age or sex. Our results highlight the patterns of adult gut biodiversity and provide a framework for future research.
Keywords: 16S rRNA amplicon; age; diversity; microbiome; sex.
Figures


References
-
- Lloyd-Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C, Giglio MG, McDonald D, Franzosa EA, Knight R, White O, Huttenhower C. 2017. Strains, functions, and dynamics in the expanded Human Microbiome Project. Nature 550:61–66. doi: 10.1038/nature23889. - DOI - PMC - PubMed
-
- McDonald D, Hyde E, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Aksenov AA, Behsaz B, Brennan C, Chen Y, DeRight Goldasich L, Dorrestein PC, Dunn RR, Fahimipour AK, Gaffney J, Gilbert JA, Gogul G, Green JL, Hugenholtz P, Humphrey G, Huttenhower C, Jackson MA, Janssen S, Jeste DV, Jiang L, Kelley ST, Knights D, Kosciolek T, Ladau J, Leach J, Marotz C, Meleshko D, Melnik AV, Metcalf JL, Mohimani H, Montassier E, Navas-Molina J, Nguyen TT, Peddada S, Pevzner P, Pollard KS, Rahnavard G, Robbins-Pianka A, Sangwan N, Shorenstein J, Smarr L, Song SJ, Spector T, Swafford AD, Thackray VG, et al. 2018. American gut: an open platform for citizen science microbiome research. mSystems 3:e00031-18. doi: 10.1128/mSystems.00031-18. - DOI - PMC - PubMed
