Synthesis and biophysical properties of carbamate-locked nucleic acid (LNA) oligonucleotides with potential antisense applications
- PMID: 31099373
- PMCID: PMC6686644
- DOI: 10.1039/c9ob00691e
Synthesis and biophysical properties of carbamate-locked nucleic acid (LNA) oligonucleotides with potential antisense applications
Abstract
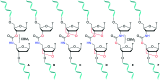
Antisense oligonucleotides (ASOs) are becoming important drugs for hard to treat diseases. Modifications to their DNA backbones are essential to inhibit degradation in vivo, but they can reduce binding affinity to RNA targets. To address this problem we have combined the enzymatic resistance of carbamate (CBM) DNA backbone analogues with the thermodynamic stability conferred by locked nucleic acid sugars (LNA). Using a dinucleotide phosphoramidite strategy and automated solid phase synthesis, we have synthesised a set of oligonucleotides modified with multiple LNA-CBM units. The LNA sugars restore binding affinity to RNA targets, and in this respect LNA position with respect to the CBM linkage is important. Oligonucleotides containing carbamate flanked on its 5'and 3'-sides by LNA form stable duplexes with RNA and unstable duplexes with DNA, which is desirable for antisense applications. Carbamate-LNA modified oligonucleotides also show increased stability in the presence of snake venom and foetal bovine serum compared to LNA or CBM backbones alone.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

