Regio- and Enantioselective Synthesis of 1,2-Diamine Derivatives by Copper-Catalyzed Hydroamination
- PMID: 31099584
- PMCID: PMC6825330
- DOI: 10.1021/acs.orglett.9b01592
Regio- and Enantioselective Synthesis of 1,2-Diamine Derivatives by Copper-Catalyzed Hydroamination
Abstract
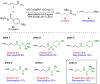
A highly regio- and enantioselective synthesis of 1,2-diamine derivatives from γ-substituted allylic pivalamides using copper-catalyzed hydroamination is reported. The N-pivaloyl group is essential, in both facilitating the hydrocupration step and suppressing an unproductive β-elimination from the alkylcopper intermediate. This approach enables an efficient construction of chiral differentially protected vicinal diamines under mild conditions with broad functional group tolerance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




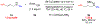
References
-
- Cho Y-H; Zunic V; Senboku H; Olsen M; Lautens M J. Am. Chem. Soc 2006, 128, 6837–6846. - PubMed
- Cho Y-H; Fayol A; Lautens M Tetrahedron: Asymmetry 2006, 17, 416–427.
- Arai K; Lucarini S; Salter MM; Ohta K; Yamashita Y; Kobayashi S J. Am. Chem. Soc 2007, 129, 8103–8111. - PubMed
- Yu R; Yamashita Y; Kobayashi S Adv. Synth. Catal 2009, 351, 147–152.
- Wu B; Gallucci JC; Parquette JR; RajanBabu TV Chem. Sci 2014, 5, 1102–1117.
- Chai Z; Yang P-J; Zhang H; Wang S; Yang G Angew. Chem., Int. Ed 2017, 56, 650–654. - PubMed
-
For representative examples of racemic aziridine opening followed by dynamic kinetic resolution, see:
- Trost BM; Fandrick DR; Brodmann T; Stiles DT Angew. Chem., Int. Ed 2007, 46, 6123–6125. - PubMed
-
-
For representative examples, see:
- Bloch R Chem. Rev 1998, 98, 1407–1438. - PubMed
- Manabe K; Oyamada H; Sugita K; Kobayashi S J. Org. Chem 1999, 64, 8054–8057.
- Hirabayashi R; Ogawa C; Sugiura M; Kobayashi S J. Am. Chem. Soc 2001, 123, 9493–9499. - PubMed
- Merino P; Delso I; Mannucci V; Tejero T Tetrahedron Lett. 2006, 47, 3311–3314.
-
-
-
For a review on asymmetric Mannich reactions with N-substituted nucleophiles, see:
- Arrayaás RG; Carretero JC Chem. Soc. Rev 2009, 38, 1940–1948. - PubMed
-
For representative examples of asymmetric Mannich reactions, see:
- Kobayashi S; Yazaki R; Seki K; Yamashita Y Angew. Chem., Int. Ed 2008, 47, 5613–5615. - PubMed
- Hernaández-Toribio J; Arrayaás RG; Carretero JC J. Am. Chem. Soc 2008, 130, 16150–16151. - PubMed
- Kano T; Sakamoto R; Akakura M; Maruoka K J. Am. Chem. Soc 2012, 134, 7516–7520. - PubMed
- Zhang W-Q; Cheng L-F; Yu J; Gong L-Z Angew. Chem., Int. Ed 2012, 51, 4085–4088. - PubMed
- Lin S; Kawato Y; Kumagai N; Shibasaki M Angew. Chem., Int. Ed 2015, 54, 5183–5186. - PubMed
- Kondo M; Nishi T; Hatanaka T; Funahashi Y; Nakamura S Angew. Chem., Int. Ed 2015, 54, 8198–8202. - PubMed
- Kano T; Kobayashi R; Maruoka K Angew. Chem., Int. Ed 2015, 54, 8471–8474. - PubMed
-
For representative examples of asymmetric vinylogous Mannich reactions, see:
- Ranieri B; Curti C; Battistini L; Sartori A; Pinna L; Casiraghi G; Zanardi F J. Org. Chem 2011, 76, 10291–10298. - PubMed
- Silverio DL; Fu P; Carswell EL; Snapper ML; Hoveyda AH Tetrahedron Lett. 2015, 56, 3489–3493. - PMC - PubMed
-
-
-
For representative examples of asymmetric nitro-Mannich reactions, see:
- Yamada K.-i.; Harwood SJ; Gröger H; Shibasaki M Angew. Chem., Int. Ed 1999, 38, 3504–3506. - PubMed
- Yamada K.-i.; Moll G; Shibasaki M Synlett 2001, 980–982.
- Knudsen KR; Risgaard T; Nishiwaki N; Gothelf KV; Jørgensen KA J. Am. Chem. Soc 2001, 123, 5843–5844. - PubMed
- Nugent BM; Yoder RA; Johnston JN J. Am. Chem. Soc 2004, 126, 3418–3419. - PubMed
- Yoon TP; Jacobsen EN Angew. Chem., Int. Ed 2005, 44, 466–468. - PubMed
- Singh A; Yoder RA; Shen B; Johnston JN J. Am. Chem. Soc 2007, 129, 3466–3467. - PubMed
- Trost BM; Lupton DW Org. Lett 2007, 9, 2023–2026. - PubMed
- Singh A; Johnston JN J. Am. Chem. Soc 2008, 130, 5866–5867. - PubMed
- Uraguchi D; Koshimoto K; Ooi T J. Am. Chem. Soc 2008, 130, 10878–10879. - PubMed
- Davis TA; Wilt JC; Johnston JN J. Am. Chem. Soc 2010, 132, 2880–2882. - PMC - PubMed
- Handa S; Gnanadesikan V; Matsunaga S; Shibasaki M J. Am. Chem. Soc 2010, 132, 4925–4934. - PubMed
- Sprague DJ; Singh A; Johnston JN Chem. Sci 2018, 9, 2336–2339. - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

