The pathogenic actinobacterium Rhodococcus equi: what's in a name?
- PMID: 31099908
- PMCID: PMC6852188
- DOI: 10.1111/mmi.14267
The pathogenic actinobacterium Rhodococcus equi: what's in a name?
Abstract
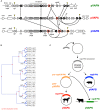
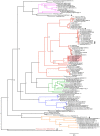
Rhodococcus equi is the only recognized animal pathogenic species within an extended genus of metabolically versatile Actinobacteria of considerable biotechnological interest. Best known as a horse pathogen, R. equi is commonly isolated from other animal species, particularly pigs and ruminants, and causes severe opportunistic infections in people. As typical in the rhodococci, R. equi niche specialization is extrachromosomally determined, via a conjugative virulence plasmid that promotes intramacrophage survival. Progress in the molecular understanding of R. equi and its recent rise as a novel paradigm of multihost adaptation has been accompanied by an unusual nomenclatural instability, with a confusing succession of names: "Prescottia equi", "Prescotella equi", Corynebacterium hoagii and Rhodococcus hoagii. This article reviews current advances in the genomics, biology and virulence of this pathogenic actinobacterium with a unique mechanism of plasmid-transferable animal host tropism. It also discusses the taxonomic and nomenclatural issues around R. equi in the light of recent phylogenomic evidence that confirms its membership as a bona fide Rhodococcus.
© 2019 The Authors. Molecular Microbiology Published by John Wiley & Sons Ltd.
Figures



References
-
- von Bargen, K. and Haas, A. (2009) Molecular and infection biology of the horse pathogen Rhodococcus equi . FEMS Microbiology Reviews, 33, 870–891. - PubMed
-
- Bryan, L.K. , Clark, S.D. , Diaz‐Delgado, J. , Lawhon, S.D. and Edwards, J.F. (2017) Rhodococcus equi infections in dogs. Veterinary Pathology, 54, 159–163. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

