Effect of intraoperative infusion of dexmedetomidine on postoperative recovery in patients undergoing endovascular interventional therapies: A prospective, randomized, controlled trial
- PMID: 31099992
- PMCID: PMC6625481
- DOI: 10.1002/brb3.1317
Effect of intraoperative infusion of dexmedetomidine on postoperative recovery in patients undergoing endovascular interventional therapies: A prospective, randomized, controlled trial
Abstract
Background: Rapid emergence from general anesthesia during endovascular interventional therapies (EITs) is important. However, the solution that improved quality of both analepsia and postoperative recovery after EITs has not been specifically addressed. We conducted this prospective, randomized, controlled trial to evaluate the intraoperative infusion of dexmedetomidine on quality of analepsia and postoperative recovery in patients undergoing EITs.
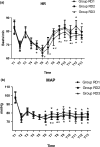
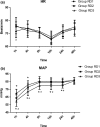
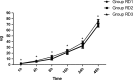
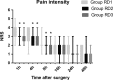
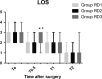
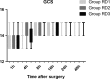
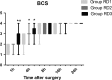
Methods: Eighty-six patients undergoing EITs were divided into three groups: RD1 (dexmedetomidine at an initial dose of 0.5 μg/kg for 10 min adjusted to 0.2 μg kg-1 hr-1 throughout EIT), RD2 (dexmedetomidine at an initial dose of 0.5 μg/kg for 10 min adjusted to 0.4 μg kg-1 hr-1 throughout EIT), and RD3 (dexmedetomidine at an initial dose of 0.5 μg/kg for 10 min adjusted to 0.6 μg kg-1 hr-1 throughout EIT). An analgesia system delivered sufentanil only. The primary outcome measure was the total consumption of nimodipine during the first 48 hr after surgery. The secondary outcome measures were sufentanil consumption, pain intensity, hemodynamics, functional activity score (FAS), neurologic examination, level of sedation (LOS), and Bruggrmann comfort scale (BCS). We also recorded the intraoperative hemodynamic data, requirement of narcotic and vasoactive drugs, prevalence of complications and symptomatic cerebral vasospasm, duration of postanesthesia care unit (PACU) stay, Glasgow Outcome Score (GOS) at 3 months, and prevalence of cerebral infarction 30 days after surgery.
Results: Dexmedetomidine application in the regimen RD3 reduced the consumption of the total dose of nimodipine and sufentanil 48 hr after surgery, prevalence of symptomatic cerebral vasospasm, consumption of narcotic drugs and nimodipine during surgery, pain intensity during the first 8 hr after surgery, and increased both BCS during the first 4 hr after surgery and hemodynamic stability. However, the LOS was increased at the 0.5 hr after surgery and surgeon satisfaction score was lower. There were no significant differences among the groups for consumption of vasoactive drugs except urapidil, Glasgow coma scale (GCS) and FAS during the first 48 hr after surgery, GOS at 3 months, and cerebral infarction after 30 days.
Conclusions: Dexmedetomidine (an initial dose of 0.5 μg/kg for 10 min adjusted to 0.6 μg kg-1 hr-1 throughout EIT) could reduce the total consumption of nimodipine and opioid during the first 48 hr after surgery, the concerning adverse effects, and improve pain scores. The optimal dosage of dexmedetomidine during EITs merits further investigation.
Keywords: cerebral vasospasm; dexmedetomidine; endovascular interventional therapies; neuroanesthesia; nimodipine.
© 2019 The Authors Brain and Behavior Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declared that the research was conducted in the absence of any commercial or financial relationships.
Figures

References
-
- Albanna, W. , Weiss, M. , Müller, M. , Brockmann, M. A. , Rieg, A. , Conzen, C. , … Schubert, G. A. (2017). Endovascular rescue therapies for refractory vasospasm after subarachnoid hemorrhage: A prospective evaluation study using multimodal, continuous event neuromonitoring. Neurosurgery, 80(6), 942–949. 10.1093/neuros/nyw132 - DOI - PubMed
-
- Amadori, A. , Franchi, M. , Consoli, A. , Mencherini, N. , Palmarini, N. , Nappini, S. , … Mangiafico, S. (2013). Cooperative patient general anesthesia (Co.Pa.Ge.A.): The new anesthetic technique for neurological monitoring of the "cooperative" patient during endovascular treatment of elective brain arteriovenous malformations with Onyx18. Journal of Neurosurgical Anesthesiology, 25(3), 335–339. 10.1097/ANA.0b013e31828bac8d - DOI - PubMed
-
- Andereggen, L. , Beck, J. , Z'Graggen, W. J. , Schroth, G. , Andres, R. H. , Murek, M. , … Gralla, J. (2017). Feasibility and safety of repeat instant endovascular interventions in patients with refractory cerebral vasospasms. American Journal of Neuroradiology, 38(3), 561–567. 10.3174/ajnr.A5024 - DOI - PMC - PubMed
-
- Baguley, I. J. , Perkes, I. E. , Fernandez‐Ortega, J.‐F. , Rabinstein, A. A. , Dolce, G. , & Hendricks, H. T. (2014). Paroxysmal sympathetic hyperactivity after acquired brain injury: Consensus on conceptual definition, nomenclature, and diagnostic criteria. Journal of Neurotrauma, 31(17), 1515–1520. 10.1089/neu.2013.3301 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

