Lipophilic statins inhibit YAP nuclear localization, co-activator activity and colony formation in pancreatic cancer cells and prevent the initial stages of pancreatic ductal adenocarcinoma in KrasG12D mice
- PMID: 31100067
- PMCID: PMC6524808
- DOI: 10.1371/journal.pone.0216603
Lipophilic statins inhibit YAP nuclear localization, co-activator activity and colony formation in pancreatic cancer cells and prevent the initial stages of pancreatic ductal adenocarcinoma in KrasG12D mice
Abstract
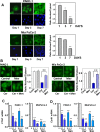
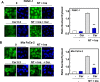
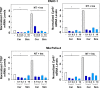
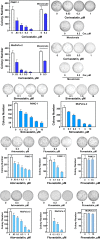
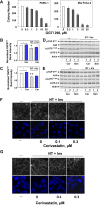
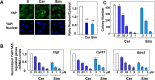
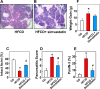
We examined the impact of statins on Yes-associated Protein (YAP) localization, phosphorylation and transcriptional activity in human and mouse pancreatic ductal adenocarcinoma (PDAC) cells. Exposure of sparse cultures of PANC-1 and MiaPaCa-2 cells to cerivastatin or simvastatin induced a striking re-localization of YAP from the nucleus to the cytoplasm and inhibited the expression of the YAP/TEAD-regulated genes Connective Tissue Growth Factor (CTGF) and Cysteine-rich angiogenic inducer 61 (CYR61). Statins also prevented YAP nuclear import and expression of CTGF and CYR61 stimulated by the mitogenic combination of insulin and neurotensin in dense culture of these PDAC cells. Cerivastatin, simvastatin, atorvastatin and fluvastatin also inhibited colony formation by PANC-1 and MiaPaCa-2 cells in a dose-dependent manner. In contrast, the hydrophilic statin pravastatin did not exert any inhibitory effect even at a high concentration (10 μM). Mechanistically, cerivastatin did not alter the phosphorylation of YAP at Ser127 in either PANC-1 or MiaPaCa-2 cells incubated without or with neurotensin and insulin but blunted the assembly of actin stress fiber in these cells. We extended these findings with human PDAC cells using primary KC and KPC cells, (expressing KrasG12D or both KrasG12D and mutant p53, respectively) isolated from KC or KPC mice. Using cultures of these murine cells, we show that lipophilic statins induced striking YAP translocation from the nucleus to the cytoplasm, inhibited the expression of Ctgf, Cyr61 and Birc5 and profoundly inhibited colony formation of these cells. Administration of simvastatin to KC mice subjected to diet-induced obesity prevented early pancreatic acini depletion and PanIN formation. Collectively, our results show that lipophilic statins restrain YAP activity and proliferation in pancreatic cancer cell models in vitro and attenuates early lesions leading to PDAC in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. . - PubMed
-
- Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12(2):81–91. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

