TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response
- PMID: 31100073
- PMCID: PMC6524796
- DOI: 10.1371/journal.pgen.1007947
TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response
Abstract
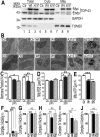
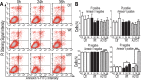
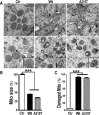
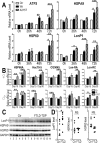
Mutations in or dys-regulation of the TDP-43 gene have been associated with TDP-43 proteinopathy, a spectrum of neurodegenerative diseases including Frontotemporal Lobar Degeneration (FTLD) and Amyotrophic Lateral Sclerosis (ALS). The underlying molecular and cellular defects, however, remain unclear. Here, we report a systematic study combining analyses of patient brain samples with cellular and animal models for TDP-43 proteinopathy. Electron microscopy (EM) analyses of patient samples revealed prominent mitochondrial impairment, including abnormal cristae and a loss of cristae; these ultrastructural changes were consistently observed in both cellular and animal models of TDP-43 proteinopathy. In these models, increased TDP-43 expression induced mitochondrial dysfunction, including decreased mitochondrial membrane potential and elevated production of reactive oxygen species (ROS). TDP-43 expression suppressed mitochondrial complex I activity and reduced mitochondrial ATP synthesis. Importantly, TDP-43 activated the mitochondrial unfolded protein response (UPRmt) in both cellular and animal models. Down-regulating mitochondrial protease LonP1 increased mitochondrial TDP-43 levels and exacerbated TDP-43-induced mitochondrial damage as well as neurodegeneration. Together, our results demonstrate that TDP-43 induced mitochondrial impairment is a critical aspect in TDP-43 proteinopathy. Our work has not only uncovered a previously unknown role of LonP1 in regulating mitochondrial TDP-43 levels, but also advanced our understanding of the pathogenic mechanisms for TDP-43 proteinopathy. Our study suggests that blocking or reversing mitochondrial damage may provide a potential therapeutic approach to these devastating diseases.
Conflict of interest statement
Authors declare that there is no competing interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

