Adolescent cognitive control, theta oscillations, and social observation
- PMID: 31100431
- PMCID: PMC7715006
- DOI: 10.1016/j.neuroimage.2019.04.077
Adolescent cognitive control, theta oscillations, and social observation
Abstract
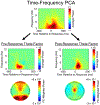
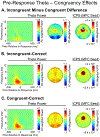
Theta oscillations (4-8 Hz) provide an organizing principle of cognitive control, allowing goal-directed behavior. In adults, theta power over medial-frontal cortex (MFC) underlies conflict/error monitoring, whereas theta connectivity between MFC and lateral-frontal regions reflects cognitive control recruitment. However, prior work has not separated theta responses that occur before and immediately after a motor response, nor explained how medial-lateral connectivity drives different kinds of control behaviors. Theta's role during adolescence, a developmental window characterized by a motivation-control mismatch also remains unclear. As social observation is known to influence motivation, this might be a particularly important context for studying adolescent theta dynamics. Here, adolescents performed a flanker task alone or under social observation. Focusing first on the nonsocial context, we parsed cognitive control into dissociable subprocesses, illustrating how theta indexes distinct components of cognitive control working together dynamically to produce goal-directed behavior. We separated theta power immediately before/after motor responses, identifying behavioral links to conflict monitoring and error monitoring, respectively. MFC connectivity was separated before/after responses and behaviorally-linked to reactive and proactive control, respectively. Finally, distinct forms of post-error control were dissociated, based on connectivity with rostral/caudal frontal cortex. Social observation was found to exclusively upregulate theta measures indexing post-response error monitoring and proactive control, as opposed to conflict monitoring and reactive control. Linking adolescent cognitive control to theta oscillations provides a bridge between non-invasive recordings in humans and mechanistic studies of neural oscillations in animal models; links to social observation provide insight into the motivation-control interactions that occur during adolescence.
Keywords: Adolescence; Cognitive control; Medial-frontal cortex (MFC); Motivation; Social observation; Theta.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests: none
Figures








References
-
- Badre D 2008. Cognitive control, hierarchy, and the rostro–caudal organization of the frontal lobes. Trends Cogn Sci. 12:193–200. - PubMed
-
- Badre D, Wagner AD. 2004. Selection, Integration, and Conflict Monitoring: Assessing the Nature and Generality of Prefrontal Cognitive Control Mechanisms. Neuron. 41:473–487. - PubMed
-
- Banich MT. 2009. Executive Function: The Search for an Integrated Account. Curr Dir Psychol Sci. 18:89–94.
-
- Benjamini Y, Hochberg Y. 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 57:289–300.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

