Antifungal Activities of 4″,6″-Disubstituted Amphiphilic Kanamycins
- PMID: 31100822
- PMCID: PMC6571828
- DOI: 10.3390/molecules24101882
Antifungal Activities of 4″,6″-Disubstituted Amphiphilic Kanamycins
Abstract
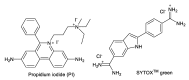
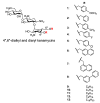
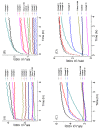
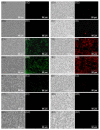
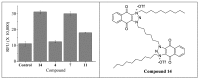
Amphiphilic kanamycins derived from the classic antibiotic kanamycin have attracted interest due to their novel bioactivities beyond inhibition of bacteria. In this study, the recently described 4″,6″-diaryl amphiphilic kanamycins reported as inhibitors of connexin were examined for their antifungal activities. Nearly all 4″,6″-diaryl amphiphilic kanamycins tested had antifungal activities comparable to those of 4″,6″-dialkyl amphiphilic kanamycins, reported previously against several fungal strains. The minimal growth inhibitory concentrations (MICs) correlated with the degree of amphiphilicity (cLogD) of the di-substituted amphiphilic kanamycins. Using the fluorogenic dyes, SYTOXTM Green and propidium iodide, the most active compounds at the corresponding MICs or at 2×MICs caused biphasic dye fluorescence increases over time with intact cells. Further lowering the concentrations to half MICs caused first-order dye fluorescence increases. Interestingly, 4×MIC or 8×MIC levels resulted in fluorescence suppression that did not correlate with the MIC and plasma membrane permeabilization. The results show that 4″,6″-diaryl amphiphilic kanamycins are antifungal and that amphiphilicity parameter cLogD is useful for the design of the most membrane-active versions. A cautionary limitation of fluorescence suppression was revealed when using fluorogenic dyes to measure cell-permeation mechanisms with these antifungals at high concentrations. Finally, 4″,6″-diaryl amphiphilic kanamycins elevate the production of cellular reactive oxygen species as other reported amphiphilic kanamycins.
Keywords: Cryptococcus neoformans; SYTOXTM green; amphiphilic kanamycin; antifungal; kinetic membrane permeabilization; propidium iodide.
Conflict of interest statement
No conflicts of interest declared.
Figures




References
-
- Donovick R., Gold W., Pagano J.F., Stout H.A. Amphotericins a and b, antifungal antibiotics produced by a streptomycete. I. In vitro studies. Antibiot. Annu. 1955;3:579–586. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

