Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release
- PMID: 31100826
- PMCID: PMC6566147
- DOI: 10.3390/ma12101610
Nano-Carriers Based on pH-Sensitive Star-Shaped Copolymers for Drug-Controlled Release
Abstract
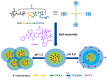
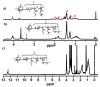
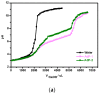
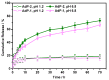
Polymeric nano-carriers are considered as promising tools in biomedical applications due to multiple attractive characteristics including their low toxicity, high loading capacity, controlled drug release capabilities, and highly tunable chemical properties. Here, a series of pH-sensitive star-shaped copolymers, Ad-P[(EMA-co-MAA)-b-PPEGMA]4, was prepared via electron transfer atom radical polymerization (ARGETE ATRP) and selective hydrolysis. These star-shaped copolymers can be self-assembled into micelles (Dh = 150-160 nm) and their critical micelle concentrations (CMC) were estimated to be 3.9-5.0 mg/L. The pH-sensitiveness of the micelles was evidenced by transmission electron microscopy (TEM) and dynamic light scattering (DLS). The maximal paclitaxel (PTX) loading efficiency (DLC) and entrapment efficiency (EE) were 18.9% and 36%, respectively. In vitro release studies revealed that about 19% of the PTX released at an acidic condition of pH 1.2 over 70 h, whereas more than 70% was released within the same time interval at pH 6.8. In vitro cytotoxicity suggested that the low cytotoxicity of the blank micelles, while the PTX-loaded micelles providing the cytotoxicity close to that of free PTX. These results indicated that this novel pH-sensitive nano-carriers have great potential applications for oral drug-controlled release.
Keywords: adamantane; drug release; nano-carrier; pH-sensitive copolymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Kowalczuk A., Trzcinska R., Trzebicka B., Müller A.H.E., Dworak A., Tsvetanov C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014;39:43–86. doi: 10.1016/j.progpolymsci.2013.10.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

