New Approaches in the Management of Sudden Cardiac Death in Patients with Heart Failure-Targeting the Sympathetic Nervous System
- PMID: 31100908
- PMCID: PMC6567277
- DOI: 10.3390/ijms20102430
New Approaches in the Management of Sudden Cardiac Death in Patients with Heart Failure-Targeting the Sympathetic Nervous System
Abstract
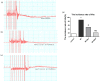
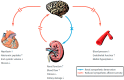
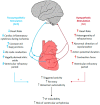
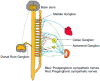
Cardiovascular diseases (CVDs) have been considered the most predominant cause of death and one of the most critical public health issues worldwide. In the past two decades, cardiovascular (CV) mortality has declined in high-income countries owing to preventive measures that resulted in the reduced burden of coronary artery disease (CAD) and heart failure (HF). In spite of these promising results, CVDs are responsible for ~17 million deaths per year globally with ~25% of these attributable to sudden cardiac death (SCD). Pre-clinical data demonstrated that renal denervation (RDN) decreases sympathetic activation as evaluated by decreased renal catecholamine concentrations. RDN is successful in reducing ventricular arrhythmias (VAs) triggering and its outcome was not found inferior to metoprolol in rat myocardial infarction model. Registry clinical data also suggest an advantageous effect of RDN to prevent VAs in HF patients and electrical storm. An in-depth investigation of how RDN, a minimally invasive and safe method, reduces the burden of HF is urgently needed. Myocardial systolic dysfunction is correlated to neuro-hormonal overactivity as a compensatory mechanism to keep cardiac output in the face of declining cardiac function. Sympathetic nervous system (SNS) overactivity is supported by a rise in plasma noradrenaline (NA) and adrenaline levels, raised central sympathetic outflow, and increased organ-specific spillover of NA into plasma. Cardiac NA spillover in untreated HF individuals can reach ~50-fold higher levels compared to those of healthy individuals under maximal exercise conditions. Increased sympathetic outflow to the renal vascular bed can contribute to the anomalies of renal function commonly associated with HF and feed into a vicious cycle of elevated BP, the progression of renal disease and worsening HF. Increased sympathetic activity, amongst other factors, contribute to the progress of cardiac arrhythmias, which can lead to SCD due to sustained ventricular tachycardia. Targeted therapies to avoid these detrimental consequences comprise antiarrhythmic drugs, surgical resection, endocardial catheter ablation and use of the implantable electronic cardiac devices. Analogous NA agents have been reported for single photon-emission-computed-tomography (SPECT) scans usage, specially the 123I-metaiodobenzylguanidine (123I-MIBG). Currently, HF prognosis assessment has been improved by this tool. Nevertheless, this radiotracer is costly, which makes the use of this diagnostic method limited. Comparatively, positron-emission-tomography (PET) overshadows SPECT imaging, because of its increased spatial definition and broader reckonable methodologies. Numerous ANS radiotracers have been created for cardiac PET imaging. However, so far, [11C]-meta-hydroxyephedrine (HED) has been the most significant PET radiotracer used in the clinical scenario. Growing data has shown the usefulness of [11C]-HED in important clinical situations, such as predicting lethal arrhythmias, SCD, and all-cause of mortality in reduced ejection fraction HF patients. In this article, we discussed the role and relevance of novel tools targeting the SNS, such as the [11C]-HED PET cardiac imaging and RDN to manage patients under of SCD risk.
Keywords: heart failure; hypertension; positron emission tomography; renal denervation; sudden cardiac death; sympathetic nervous system; ventricular arrhythmias.
Conflict of interest statement
M.G.K., J.M.N., H.V., R.C., J.J.S.-Y.C., L.M.L.-G., J.K.H., V.B.M. and G.D. declare that they have no conflict of interest. M.P.S. is supported by an NHMRC Research Fellowship and has received consulting fees, and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer-Ingelheim. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Radionuclide imaging of cardiac sympathetic innervation in heart failure: unlocking untapped potential.Heart Fail Rev. 2015 Mar;20(2):215-26. doi: 10.1007/s10741-014-9456-5. Heart Fail Rev. 2015. PMID: 25135163 Review.
-
Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction.Circ Cardiovasc Imaging. 2010 Sep;3(5):595-603. doi: 10.1161/CIRCIMAGING.109.920538. Epub 2010 Jun 9. Circ Cardiovasc Imaging. 2010. PMID: 20534790
-
Recent Advances and Clinical Applications of PET Cardiac Autonomic Nervous System Imaging.Curr Cardiol Rep. 2017 Apr;19(4):33. doi: 10.1007/s11886-017-0843-0. Curr Cardiol Rep. 2017. PMID: 28321682 Free PMC article. Review.
-
Early therapeutic effects of adaptive servo-ventilation on cardiac sympathetic nervous function in patients with heart failure evaluated using a combination of 11C-HED PET and 123I-MIBG SPECT.J Nucl Cardiol. 2019 Aug;26(4):1079-1089. doi: 10.1007/s12350-017-1132-4. Epub 2017 Nov 27. J Nucl Cardiol. 2019. PMID: 29181786 Free PMC article.
-
Regional Cardiac Sympathetic Nervous System Evaluation Using 123I-mIBG SPECT in Patients with Heart Failure.J Med Imaging Radiat Sci. 2018 Dec;49(4):397-405. doi: 10.1016/j.jmir.2018.05.001. Epub 2018 Jun 19. J Med Imaging Radiat Sci. 2018. PMID: 30514557
Cited by
-
Catestatin as a Biomarker of Cardiovascular Diseases: A Clinical Perspective.Biomedicines. 2021 Nov 25;9(12):1757. doi: 10.3390/biomedicines9121757. Biomedicines. 2021. PMID: 34944578 Free PMC article. Review.
-
Editorial for the IJMS Special Issue "Progress in Understanding of Cardiac Arrhythmia Mechanisms and Antiarrhythmic Targets".Int J Mol Sci. 2023 May 23;24(11):9134. doi: 10.3390/ijms24119134. Int J Mol Sci. 2023. PMID: 37298084 Free PMC article.
-
Stellate Ganglia and Cardiac Sympathetic Overactivation in Heart Failure.Int J Mol Sci. 2022 Nov 1;23(21):13311. doi: 10.3390/ijms232113311. Int J Mol Sci. 2022. PMID: 36362099 Free PMC article. Review.
-
Neurohumoral Activation in Heart Failure.Int J Mol Sci. 2023 Oct 23;24(20):15472. doi: 10.3390/ijms242015472. Int J Mol Sci. 2023. PMID: 37895150 Free PMC article. Review.
-
Machine learning powered tools for automated analysis of muscle sympathetic nerve activity recordings.Physiol Rep. 2021 Aug;9(16):e14996. doi: 10.14814/phy2.14996. Physiol Rep. 2021. PMID: 34427381 Free PMC article.
References
-
- Alwan A. Global Status Report on Noncommunicable Diseases 2010. World Health Organization; Geneva, Switzerland: 2011. Global status report on noncommunicable diseases 2010: Introduction; pp. vii–ix.
-
- Sacco R.L., Roth G.A., Reddy K.S., Arnett D.K., Bonita R., Gaziano T.A., Heidenreich P.A., Huffman M.D., Mayosi B.M., Mendis S., et al. The Heart of 25 by 25: Achieving the Goal of Reducing Global and Regional Premature Deaths from Cardiovascular Diseases and Stroke: A Modeling Study from the American Heart Association and World Heart Federation. Circulation. 2016;133:e674–e690. doi: 10.1161/CIR.0000000000000395. - DOI - PubMed
-
- Niemeijer M.N., van den Berg M.E., Leening M.J., Hofman A., Franco O.H., Deckers J.W., Heeringa J., Rijnbeek P.R., Stricker B.H., Eijgelsheim M., et al. Declining incidence of sudden cardiac death from 1990-2010 in a general middle-aged and elderly population: The Rotterdam Study. Heart Rhythm. 2015;12:123–129. doi: 10.1016/j.hrthm.2014.09.054. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

