A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control
- PMID: 31100920
- PMCID: PMC6572709
- DOI: 10.3390/ijerph16101736
A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control
Abstract
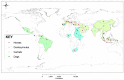
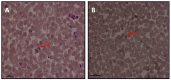
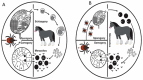
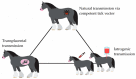
Equine Piroplasmosis (EP) is a tick-borne disease caused by apicomplexan protozoan parasites, Babesia caballi and Theileria equi. The disease is responsible for serious economic losses to the equine industry. It principally affects donkeys, horses, mules, and zebra but DNA of the parasites has also been detected in dogs and camels raising doubt about their host specificity. The disease is endemic in tropical and temperate regions of the world where the competent tick vectors are prevalent. Infected equids remain carrier for life with T. equi infection, whilst, infection with B. caballi is cleared within a few years. This review focuses on all aspects of the disease from the historical overview, biology of the parasite, epidemiology of the disease (specifically highlighting other non-equine hosts, such as dogs and camels), vector, clinical manifestations, risk factors, immunology, genetic diversity, diagnosis, treatment, and prevention.
Keywords: Babesia caballi; Equine Piroplasmosis; Theileria equi; epidemiology; equines; tick-borne disease; ticks.
Conflict of interest statement
No conflict of interest exists among the authors.
Figures
References
-
- Sumbria D., Moudgil A.D., Singla L.D. Equine Piroplasmosis: Current status. Veterinaria. 2014;1:9–14.
-
- Rothschild C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013;33:497–508. doi: 10.1016/j.jevs.2013.03.189. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

