Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome
- PMID: 31100966
- PMCID: PMC6562906
- DOI: 10.3390/cells8050467
Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome
Abstract
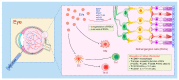
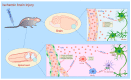
Mesenchymal stem cell (MSC)-sourced secretome, defined as the set of MSC-derived bioactive factors (soluble proteins, nucleic acids, lipids and extracellular vesicles), showed therapeutic effects similar to those observed after transplantation of MSCs. MSC-derived secretome may bypass many side effects of MSC-based therapy, including unwanted differentiation of engrafted MSCs. In contrast to MSCs which had to be expanded in culture to reach optimal cell number for transplantation, MSC-sourced secretome is immediately available for treatment of acute conditions, including fulminant hepatitis, cerebral ischemia and myocardial infarction. Additionally, MSC-derived secretome could be massively produced from commercially available cell lines avoiding invasive cell collection procedure. In this review article we emphasized molecular and cellular mechanisms that were responsible for beneficial effects of MSC-derived secretomes in the treatment of degenerative and inflammatory diseases of hepatobiliary, respiratory, musculoskeletal, gastrointestinal, cardiovascular and nervous system. Results obtained in a large number of studies suggested that administration of MSC-derived secretomes represents a new, cell-free therapeutic approach for attenuation of inflammatory and degenerative diseases. Therapeutic effects of MSC-sourced secretomes relied on their capacity to deliver genetic material, growth and immunomodulatory factors to the target cells enabling activation of anti-apoptotic and pro-survival pathways that resulted in tissue repair and regeneration.
Keywords: degenerative diseases; inflammatory diseases; mesenchymal stem cells; secretome; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kuriyan A.E., Albini T.A., Townsend J.H., Rodriguez M., Pandya H.K., Leonard R.E., 2nd, Parrott M.B., Rosenfeld P.J., Flynn H.W., Jr., Goldberg J.L. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med. 2017;376:1047–1053. doi: 10.1056/NEJMoa1609583. - DOI - PMC - PubMed
-
- Glassberg M.K., Minkiewicz J., Toonkel R.L., Simonet E.S., Rubio G.A., DiFede D., Shafazand S., Khan A., Pujol M.V., LaRussa V.F., et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest. 2017;151:971–981. doi: 10.1016/j.chest.2016.10.061. - DOI - PMC - PubMed
-
- Lindsay J.O., Allez M., Clark M., Labopin M., Ricart E., Rogler G., Rovira M., Satsangi J., Farge D., Hawkey C.J., ASTIC Trial Group. European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party. European Crohn’s and Colitis Organisation Autologous stem-cell transplantation in treatment-refractory Crohn’s disease: An analysis of pooled data from the ASTIC trial. Lancet Gastroenterol. Hepatol. 2017;2:399–406. doi: 10.1016/S2468-1253(17)30056-0. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

