Role of the Hippo Pathway in Fibrosis and Cancer
- PMID: 31100975
- PMCID: PMC6562634
- DOI: 10.3390/cells8050468
Role of the Hippo Pathway in Fibrosis and Cancer
Abstract
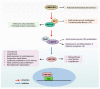
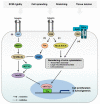
The Hippo pathway is the key player in various signaling processes, including organ development and maintenance of tissue homeostasis. This pathway comprises a core kinases module and transcriptional activation module, representing a highly conserved mechanism from Drosophila to vertebrates. The central MST1/2-LATS1/2 kinase cascade in this pathway negatively regulates YAP/TAZ transcription co-activators in a phosphorylation-dependent manner. Nuclear YAP/TAZ bind to transcription factors to stimulate gene expression, contributing to the regenerative potential and regulation of cell growth and death. Recent studies have also highlighted the potential role of Hippo pathway dysfunctions in the pathology of several diseases. Here, we review the functional characteristics of the Hippo pathway in organ fibrosis and tumorigenesis, and discuss its potential as new therapeutic targets.
Keywords: ECM; EMT; LATS1/2; MST1/2; TAZ; YAP; cancer; fibrosis; myofibroblast.
Conflict of interest statement
The author declares that there is no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

