A deficiency in SUMOylation activity disrupts multiple pathways leading to neural tube and heart defects in Xenopus embryos
- PMID: 31101013
- PMCID: PMC6525467
- DOI: 10.1186/s12864-019-5773-3
A deficiency in SUMOylation activity disrupts multiple pathways leading to neural tube and heart defects in Xenopus embryos
Abstract
Background: Adenovirus protein, Gam1, triggers the proteolytic destruction of the E1 SUMO-activating enzyme. Microinjection of an empirically determined amount of Gam1 mRNA into one-cell Xenopus embryos can reduce SUMOylation activity to undetectable, but nonlethal, levels, enabling an examination of the role of this post-translational modification during early vertebrate development.
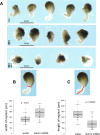
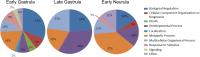
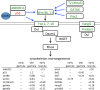
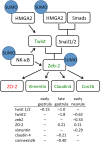
Results: We find that SUMOylation-deficient embryos consistently exhibit defects in neural tube and heart development. We have measured differences in gene expression between control and embryos injected with Gam1 mRNA at three developmental stages: early gastrula (immediately following the initiation of zygotic transcription), late gastrula (completion of the formation of the three primary germ layers), and early neurula (appearance of the neural plate). Although changes in gene expression are widespread and can be linked to many biological processes, three pathways, non-canonical Wnt/PCP, snail/twist, and Ets-1, are especially sensitive to the loss of SUMOylation activity and can largely account for the predominant phenotypes of Gam1 embryos. SUMOylation appears to generate different pools of a given transcription factor having different specificities with this post-translational modification involved in the regulation of more complex, as opposed to housekeeping, processes.
Conclusions: We have identified changes in gene expression that underlie the neural tube and heart phenotypes resulting from depressed SUMOylation activity. Notably, these developmental defects correspond to the two most frequently occurring congenital birth defects in humans, strongly suggesting that perturbation of SUMOylation, either globally or of a specific protein, may frequently be the origin of these pathologies.
Keywords: Congenital birth defects; Heart development; Neural tube development; Non-canonical Wnt signaling; Planar cell polarity; SUMO.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

