Functional capacity of natural killer cells in HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients
- PMID: 31101076
- PMCID: PMC6525417
- DOI: 10.1186/s12879-019-4032-1
Functional capacity of natural killer cells in HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients
Abstract
Background: Natural killer (NK) cells are part of the innate immune system and provide surveillance against viruses and cancers. The ability of NK cells to kill virus-infected cells depends on the balance between the effects of inhibitory and activating NK cell receptors. This study aimed to investigate the phenotypic profile and the functional capacity of NK cells in the context of HTLV-1 infection.
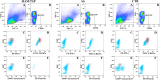
Methods: This cross-sectional study sequentially recruited HTLV-1 infected individuals with HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) and asymptomatic HTLV-1 (AS) from the Integrated and Multidisciplinary HTLV Center in Salvador, Brazil. Blood samples from healthy blood donors served as controls. NK cell surface receptors (NKG2D, KIR2DL2/KIR2DL3, NKp30, NKG2A, NKp46, TIM-3 and PD-1), intracellular cytolytic (Granzyme B, perforin) and functional markers (CD107a for degranulation, IFN-γ) were assayed by flow cytometry in the presence or absence of standard K562 target cells. In addition, cytotoxicity assays were performed in the presence or absence of anti-NKp30.
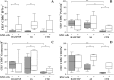
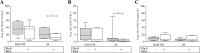
Results: The frequency of NKp30+ NK cells was significantly decreased in HAM/TSP patients [58%, Interquartile Range (IQR) 30-61] compared to controls (73%, IQR 54-79, p = 0.04). The production of cytolytic (perforin, granzyme B) and functional markers (CD107a and IFN-γ) was higher in unstimulated NK cells from HAM/TSP and AS patients compared to controls. By contrast, stimulation with K562 target cells did not alter the frequency of CD107a+ NK cells in HAM/TSP subjects compared to the other groups. Blockage of the NKp30 receptor was shown to decrease cytotoxic activity (CD107a) and IFN-γ expression only in asymptomatic HTLV-1-infected individuals.
Conclusions: NK cells from individuals with a diagnosis of HAM/TSP present decreased expression of the activating receptor NKp30, in addition to elevated degranulation activity that remained unaffected after blocking the NKp30 receptor.
Keywords: CD107; HAM/TSP; HTLV-1; NK cells; NKp30; Natural cytotoxicity receptor.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The Role of NK Cells in the Control of Viral Infection in HTLV-1 Carriers.J Immunol Res. 2019 Feb 28;2019:6574828. doi: 10.1155/2019/6574828. eCollection 2019. J Immunol Res. 2019. PMID: 30944834 Free PMC article.
-
Altered expression of degranulation-related genes in CD8+ T cells in human T lymphotropic virus type I infection.AIDS Res Hum Retroviruses. 2013 May;29(5):826-36. doi: 10.1089/aid.2012.0205. Epub 2013 Feb 5. AIDS Res Hum Retroviruses. 2013. PMID: 23301858
-
Natural killer (NK) cells in HTLV-I-associated myelopathy/tropical spastic paraparesis-decrease in NK cell subset populations and activity in HTLV-I seropositive individuals.J Neuroimmunol. 1991 Aug;33(2):121-8. doi: 10.1016/0165-5728(91)90056-d. J Neuroimmunol. 1991. PMID: 2066395
-
HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP): the role of HTLV-I-infected Th1 cells in the pathogenesis, and therapeutic strategy.Folia Neuropathol. 2009;47(2):182-94. Folia Neuropathol. 2009. PMID: 19618340 Review.
-
[Immunopathogenesis and treatment of the myelopathy associated to the HTLV-I virus].Rev Neurol. 2009 Feb 1-15;48(3):147-55. Rev Neurol. 2009. PMID: 19206063 Review. Spanish.
Cited by
-
Exploring structured molecular landscape from single-cell multi-omics data by an explainable multimodal model.iScience. 2024 Nov 2;27(12):111131. doi: 10.1016/j.isci.2024.111131. eCollection 2024 Dec 20. iScience. 2024. PMID: 39618495 Free PMC article.
-
Immunoprofiling of fresh HAM/TSP blood samples shows altered innate cell responsiveness.PLoS Negl Trop Dis. 2021 Nov 12;15(11):e0009940. doi: 10.1371/journal.pntd.0009940. eCollection 2021 Nov. PLoS Negl Trop Dis. 2021. PMID: 34767551 Free PMC article.
-
Comprehensive Insight into the Functional Roles of NK and NKT Cells in HTLV-1-Associated Diseases and Asymptomatic Carriers.Mol Neurobiol. 2024 Oct;61(10):7877-7889. doi: 10.1007/s12035-024-03999-8. Epub 2024 Mar 4. Mol Neurobiol. 2024. PMID: 38436833 Review.
-
Multi-cohort cross-omics analysis reveals disease mechanisms and therapeutic targets in HTLV-1-associated myelopathy, a neglected retroviral neuroinflammatory disorder.Res Sq [Preprint]. 2025 Apr 29:rs.3.rs-5960764. doi: 10.21203/rs.3.rs-5960764/v1. Res Sq. 2025. PMID: 40343334 Free PMC article. Preprint.
-
Mycophenolate mofetil for the long-term treatment of HTLV-1 associated myelopathy: A case report.J Neuroimmunol. 2024 Mar 15;388:578294. doi: 10.1016/j.jneuroim.2024.578294. Epub 2024 Jan 22. J Neuroimmunol. 2024. PMID: 38306927 Free PMC article.
References
-
- Guerreiro J, Santos S, Morgan D, Porto A, Muniz A, Ho J, et al. Levels of serum chemokines discriminate clinical myelopathy associated with human T lymphotropic virus type 1 (HTLV-1)/tropical spastic paraparesis (HAM/TSP) disease from HTLV-1 carrier state. Clin Exp Immunol. 2006;145(2):296–301. doi: 10.1111/j.1365-2249.2006.03150.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

