Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD
- PMID: 31101403
- PMCID: PMC10077840
- DOI: 10.1053/j.ajkd.2019.03.417
Safety and Effectiveness of Bexagliflozin in Patients With Type 2 Diabetes Mellitus and Stage 3a/3b CKD
Abstract
Rationale & objective: Hyperglycemia exacerbates the progression of chronic kidney disease (CKD), but most glucose-lowering therapies do not address morbidities associated with CKD. Sodium/glucose cotransporter 2 (SGLT2) inhibitors offer potential benefits to patients with diabetes and CKD, but their effectiveness may be diminished with decreased kidney function. We aimed to evaluate the safety and effectiveness of bexagliflozin, a novel SGLT2 inhibitor, in patients with type 2 diabetes and CKD.
Study design: Phase 3, double-blind, placebo-controlled, multicenter, multinational, randomized trial.
Setting & participants: 54 sites across 4 countries. Patients with CKD stage 3a or 3b, type 2 diabetes mellitus, and hemoglobin A1c level of 7.0% to 10.5% and estimated glomerular filtration rate (eGFR) of 30 to 59mL/min/1.73m2 who were taking oral hypoglycemic agents for 8 weeks.
Interventions: Bexagliflozin, 20mg, daily versus placebo for 24 weeks.
Outcomes: Primary outcome was change in percent hemoglobin A1c from baseline to week 24. Secondary end points included changes in body weight, systolic blood pressure, albuminuria, and hemoglobin A1c level stratified by CKD stage.
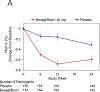
Results: 312 patients across 54 sites were analyzed. Bexagliflozin lowered hemoglobin A1c levels by 0.37% (95% CI, 0.20%-0.54%); P<0.001 compared to placebo. Patients with CKD stages 3a (eGFR, 45-<60mL/min/1.73m2) and 3b (eGFR, 30-<45mL/min/1.73m2) experienced reductions in hemoglobin A1c levels of 0.31% (P=0.007) and 0.43% (P=0.002), respectively. Bexagliflozin decreased body weight (1.61kg; P<0.001), systolic blood pressure (3.8mm Hg; P=0.02), fasting plasma glucose level (0.76mmol/L; P=0.003), and albuminuria (geometric mean ratio reduction of 20.1%; P=0.03). Urinary tract infection and genital mycotic infections were more common in the bexagliflozin group; otherwise, frequencies of adverse events were comparable between groups.
Limitations: Not designed to evaluate the impact of treatment on long-term kidney disease and cardiovascular outcomes.
Conclusions: Bexagliflozin reduces hemoglobin A1c levels in patients with diabetes and stage 3a/3b CKD and appears to be well tolerated. Additional observed benefits included reductions in body weight, systolic blood pressure, and albuminuria.
Funding: Trial was sponsored by Theracos Sub, LLC.
Keywords: SGLT2 inhibitor; Type 2 diabetes; albuminuria; bexagliflozin; chronic kidney disease (CKD); estimated glomerular filtration rate (eGFR); glucose-lowering therapy; hemoglobin A(1c) (HbA(1c)); kidney function; randomized controlled trial (RCT); renal impairment.
Copyright © 2019 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

