A sulfur and nitrogen cycle informed model to simulate nitrate treatment of reservoir souring
- PMID: 31101870
- PMCID: PMC6525193
- DOI: 10.1038/s41598-019-44033-5
A sulfur and nitrogen cycle informed model to simulate nitrate treatment of reservoir souring
Abstract
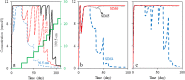
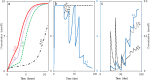
Nitrate treatment has been widely used in various seawater injection projects to treat biologic sulfate reduction or reservoir souring. To design a promising nitrate treatment plan, it is essential to have a comprehensive understanding of reactions that represent the microbial communities of the reservoir and mechanisms through which the souring process is inhibited. We employ a new approach of evaluating different reaction pathways to design reaction models that reflect governing microbial processes in a set of batch and flow experiments. Utilizing the designed models, we suggest dissimilatory nitrate reduction to ammonium is the main reaction pathway. Additionally, we illustrate nitrite inhibition is the major mechanism of nitrate treatment process; independent of nitrate reduction being autotrophic or heterotrophic. We introduce an inhibitory nitrate injection concentration that can inhibit souring regardless of nitrite inhibition effect and the distance between injection and production wells. Furthermore, we demonstrate that the ratio of the nitrite-nitrate reduction rate can be used to estimate nitrate treatment effectiveness. Our findings in regard to importance of nitrite inhibition mechanism and the inhibitory nitrate concentration are in accordance with the field observations.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ligthelm, D., De Boer, R., Brint, J. & Schulte, W. Reservoir souring: an analytical model for H2S generation and transportation in an oil reservoir owing to bacterial activity. In Offshore Europe, SPE 23141. at https://www.onepetro.org/conference-paper/SPE-23141-MS (Society of Petroleum Engineers, 1991).
-
- Sunde, E., Thorstenson, T., Torsvik, T., Vaag, J. & Espedal, M. Field-related mathematical model to predict and reduce reservoir souring. In SPE International Symposium on Oilfield Chemistry, SPE-25197-MS at https://www.onepetro.org/conference-paper/SPE-25197-MS (Society of Petroleum Engineers, 1993).
-
- Al-Rasheedi, S., Kalli, C., Thrasher, D. & Al-Qabandi, S. Prediction and evaluation of the impact of reservoir souring in North Kuwait, a case study. In Middle East Oil Show and Conference, SPE-53164-MS at https://www.onepetro.org/conference-paper/SPE-53164-MS (Society of Petroleum Engineers, 1999).
-
- Vance, I. & Thrasher, D. R. Reservoir souring: mechanisms and prevention in Petroleum microbiology (eds Ollivier, B. & Magot, M.) 123–142 (American Society of Microbiology, 2005).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

