The effect of UV-protected ethylene vinyl acetate (EVA) bags on the physicochemical stability of pediatric parenteral nutrition admixtures
- PMID: 31102140
- PMCID: PMC6593020
- DOI: 10.1007/s40199-019-00270-7
The effect of UV-protected ethylene vinyl acetate (EVA) bags on the physicochemical stability of pediatric parenteral nutrition admixtures
Abstract
Background: The safe administration of parenteral admixtures should be considered under the headings of physical and chemical stability. Vitamins are considered to be most susceptible to chemical degradation.
Objectives: To evaluate the protective effect of UV-protected monolayer ethylene vinyl acetate (EVA) bags in comparison with that of EVA bags without UV protection, on the physicochemical characteristics and stability of the light sensitive vitamins in pediatric parenteral admixtures stored under various temperature and light conditions.
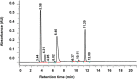
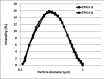
Methods: Four different parenteral pediatric admixtures (with trace elements and vitamins) in two types of ethylenovinylacetate (EVA) monolayer containers (with - yellow one and without - transparent one UV protection) were assessed. The physicochemical analyses such as visual inspection, pH and potential zeta measurements, lipid globules size distribution and vitamins concentration were performed at 0 h, 24 h, 8 days and 8 days+24 h after the preparation of the TPN admixtures. In order to quantify ascorbic acid, thiamine and pyridoxine levels, HPLC was used.
Results: No differences (p < 0.05) in physicochemical stability of TPN admixtures were noted between two types of EVA bags, with the compositions assessed; stored 8 days (4 °C ± 2) without light plus 24 h at room temperature and light exposure. However significant differences were noticed in ascorbic acid, thiamine and pyridoxine content after 8 days+24 h in comparison with t = 0. This was noted for both for UV-protected bags and bags without UV-protection, Nevertheless, amounts were still within the pharmacopeial range.
Conclusions: Both EVA bags under test (with and without UV-protection) ensure physicochemical stability up 8 days at 4 °C ± 2 °C without light exposure and then 24 h at room temperature with light exposure for the total pediatric parenteral admixtures, intended for home parenteral nutrition. Graphical abstract Scheme of physicochemical analysis of parenteral admixtures.
Keywords: Ethylene vinyl acetate bags; Pediatric parenteral nutrition; Physicochemical stability; Stability of vitamins; UV-protected bags.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evaluation of physical stability of all in one parenteral admixtures for pediatric home care with high electrolytes concentrations.Nutr Hosp. 2014 Jan 1;31(1):236-43. doi: 10.3305/nh.2015.31.1.7965. Nutr Hosp. 2014. PMID: 25561115
-
Finding new solutions in pediatric parenteral admixtures: how to improve quality and to deal with shortages.Nutr Hosp. 2014 Jul 1;30(1):84-93. doi: 10.3305/nh.2014.30.1.7500. Nutr Hosp. 2014. PMID: 25137266
-
Physical characteristics of total parenteral nutrition bags significantly affect the stability of vitamins C and B1: a controlled prospective study.JPEN J Parenter Enteral Nutr. 2002 Sep-Oct;26(5):310-6. doi: 10.1177/0148607102026005310. JPEN J Parenter Enteral Nutr. 2002. PMID: 12216712
-
Practical aspects of multichamber bags for total parenteral nutrition.Curr Opin Clin Nutr Metab Care. 2005 May;8(3):291-5. doi: 10.1097/01.mco.0000165008.47073.ba. Curr Opin Clin Nutr Metab Care. 2005. PMID: 15809532 Review.
-
Compatibility and stability of additives in parenteral nutrition admixtures.Nutrition. 1998 Sep;14(9):697-706. doi: 10.1016/s0899-9007(98)00063-x. Nutrition. 1998. PMID: 9760591 Review.
Cited by
-
The Modern Approach to Total Parenteral Nutrition: Multidirectional Therapy Perspectives with a Focus on the Physicochemical Stability of the Lipid Fraction.Nutrients. 2025 Feb 28;17(5):846. doi: 10.3390/nu17050846. Nutrients. 2025. PMID: 40077716 Free PMC article. Review.
-
Data Mining-Based Stability and Prescription Analysis of Neonatal Parenteral Nutrition Solution.J Healthc Eng. 2021 Nov 30;2021:5744656. doi: 10.1155/2021/5744656. eCollection 2021. J Healthc Eng. 2021. Retraction in: J Healthc Eng. 2023 Jul 12;2023:9754791. doi: 10.1155/2023/9754791. PMID: 34900196 Free PMC article. Retracted.
References
-
- Uccello-Barrettaa G, Balzanoa F, Aielloa F, Falugiania N, Desiderib I. Stability of hydrophilic vitamins mixtures in the presence of electrolytes and trace elements for parenteral nutrition: a nuclear magnetic resonance spectroscopy investigation. J Pharm Biomed Anal. 2015;107:7–10. doi: 10.1016/j.jpba.2014.12.008. - DOI - PubMed
-
- Lobo BW, da Veiga VW, Cabral LM, Michel RC, Volpato NM, de Sousa VP. Influence of the relative composition of trace elements and vitamins in physicochemical stability of total parenteral nutrition formulations for neonatal use. Nutr J. 2012;11:26–38. doi: 10.1186/1475-2891-11-26. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

