HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity
- PMID: 31102185
- PMCID: PMC6750988
- DOI: 10.1007/s13365-019-00757-8
HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity
Abstract
Poor antiretroviral penetration may contribute to human immunodeficiency virus (HIV) persistence within the brain and to neurocognitive deficits in opiate abusers. To investigate this problem, HIV-1 Tat protein and morphine effects on blood-brain barrier (BBB) permeability and drug brain penetration were explored using a conditional HIV-1 Tat transgenic mouse model. Tat and morphine effects on the leakage of fluorescently labeled dextrans (10-, 40-, and 70-kDa) into the brain were assessed. To evaluate effects on antiretroviral brain penetration, Tat+ and Tat- mice received three antiretroviral drugs (dolutegravir, abacavir, and lamivudine) with or without concurrent morphine exposure. Antiretroviral and morphine brain and plasma concentrations were determined by LC-MS/MS. Morphine exposure, and, to a lesser extent, Tat, significantly increased tracer leakage from the vasculature into the brain. Despite enhanced BBB breakdown evidenced by increased tracer leakiness, morphine exposure led to significantly lower abacavir concentrations within the striatum and significantly less dolutegravir within the hippocampus and striatum (normalized to plasma). P-glycoprotein, an efflux transporter for which these drugs are substrates, expression and function were significantly increased in the brains of morphine-exposed mice compared to mice not exposed to morphine. These findings were consistent with lower antiretroviral concentrations in brain tissues examined. Lamivudine concentrations were unaffected by Tat or morphine exposure. Collectively, our investigations indicate that Tat and morphine differentially alter BBB integrity. Morphine decreased brain concentrations of specific antiretroviral drugs, perhaps via increased expression of the drug efflux transporter, P-glycoprotein.
Keywords: Abacavir; Dolutegravir; Lamivudine; Morphine-3-β-glucuronide; Neuro-human immunodeficiency virus (neuroHIV); P-glycoprotein; Paracellular transport; Transcellular transport; Zonula occludens-1.
Figures
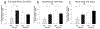

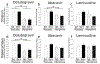
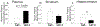
Similar articles
-
Effects of HIV-1 Tat and Methamphetamine on Blood-Brain Barrier Integrity and Function In Vitro.Antimicrob Agents Chemother. 2017 Nov 22;61(12):e01307-17. doi: 10.1128/AAC.01307-17. Print 2017 Dec. Antimicrob Agents Chemother. 2017. PMID: 28893794 Free PMC article.
-
Cell-type specific differences in antiretroviral penetration and the effects of HIV-1 Tat and morphine among primary human brain endothelial cells, astrocytes, pericytes, and microglia.Neurosci Lett. 2019 Nov 1;712:134475. doi: 10.1016/j.neulet.2019.134475. Epub 2019 Sep 3. Neurosci Lett. 2019. PMID: 31491466 Free PMC article.
-
Differential effects of fentanyl compared to morphine on neuroinflammatory signaling in the brain in EcoHIV-infected mice.J Neurovirol. 2025 Jun;31(3):242-261. doi: 10.1007/s13365-025-01252-z. Epub 2025 May 30. J Neurovirol. 2025. PMID: 40447886 Free PMC article.
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
-
Dolutegravir for the treatment of adult patients with HIV-1 infection.Expert Rev Anti Infect Ther. 2014 May;12(5):535-44. doi: 10.1586/14787210.2014.907525. Epub 2014 Apr 2. Expert Rev Anti Infect Ther. 2014. PMID: 24694091 Review.
Cited by
-
The effect of veno-arterial extracorporeal oxygenation and nasogastric tube administration on the pharmacokinetic profile of abacavir, lamivudine and dolutegravir: a case report.Antivir Ther. 2020;25(2):115-119. doi: 10.3851/IMP3355. Antivir Ther. 2020. PMID: 32341207 Free PMC article.
-
Effect of Opioid Use on Immune Activation and HIV Persistence on ART.J Neuroimmune Pharmacol. 2020 Dec;15(4):643-657. doi: 10.1007/s11481-020-09959-y. Epub 2020 Sep 25. J Neuroimmune Pharmacol. 2020. PMID: 32974750 Free PMC article. Review.
-
Morphine pharmacokinetics and opioid transporter expression at the blood-retina barrier of male and female mice.Front Pharmacol. 2023 Jun 14;14:1206104. doi: 10.3389/fphar.2023.1206104. eCollection 2023. Front Pharmacol. 2023. PMID: 37388441 Free PMC article.
-
The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era.Brain Res. 2019 Dec 1;1724:146426. doi: 10.1016/j.brainres.2019.146426. Epub 2019 Aug 29. Brain Res. 2019. PMID: 31473221 Free PMC article. Review.
-
Expression of Human Immunodeficiency Virus Transactivator of Transcription (HIV-Tat1-86) Protein Alters Nociceptive Processing that is Sensitive to Anti-Oxidant and Anti-Inflammatory Interventions.J Neuroimmune Pharmacol. 2022 Jun;17(1-2):152-164. doi: 10.1007/s11481-021-09985-4. Epub 2021 Feb 22. J Neuroimmune Pharmacol. 2022. PMID: 33619645 Free PMC article.
References
-
- Abbott NJ, Patabendige AAK, Dolman DEM, et al. (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25 - PubMed
-
- Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53 - PubMed
-
- Achanti S, Katta RR (2017) Corrigendum to “High-throughput liquid chromatography tandem mass spectrometry method for simultaneous determination of fampridine, paroxetine, and quinidine in rat plasma: Application to in vivo perfusion study” [J Food Drug Anal 24 (2016) 866–875]. J food drug Anal 25:1008. doi: 10.1016/j.jfda.2017.01.006 - DOI - PMC - PubMed
-
- Andras IE, Pu H, Deli MA, et al. (2003) HIV-1 Tat Protein Alters Tight Junction Protein Expression and Distribution in Cultured Brain Endothelial Cells. J Neurosci Res 74:255–265 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA018633/DA/NIDA NIH HHS/United States
- R00 DA039791/DA/NIDA NIH HHS/United States
- R01 DA045588/DA/NIDA NIH HHS/United States
- R21 DA045630/DA/NIDA NIH HHS/United States
- R25 MH080661/MH/NIMH NIH HHS/United States
- R01 DA044855/DA/NIDA NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- K02 DA027374/DA/NIDA NIH HHS/United States
- UL1 TR002649/TR/NCATS NIH HHS/United States
- R01 DA044939/DA/NIDA NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- R01 DA033200/DA/NIDA NIH HHS/United States
- R01 DA034231/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

