Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?
- PMID: 31102428
- PMCID: PMC6609813
- DOI: 10.1111/cas.14069
Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?
Abstract
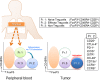
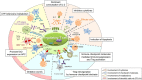
Regulatory T (Treg) cells suppress abnormal/excessive immune responses to self- and nonself-antigens to maintain immune homeostasis. In tumor immunity, Treg cells are involved in tumor development and progression by inhibiting antitumor immunity. There are several Treg cell immune suppressive mechanisms: inhibition of costimulatory signals by CD80 and CD86 expressed by dendritic cells through cytotoxic T-lymphocyte antigen-4, interleukin (IL)-2 consumption by high-affinity IL-2 receptors with high CD25 (IL-2 receptor α-chain) expression, secretion of inhibitory cytokines, metabolic modulation of tryptophan and adenosine, and direct killing of effector T cells. Infiltration of Treg cells into the tumor microenvironment (TME) occurs in multiple murine and human tumors. Regulatory T cells are chemoattracted to the TME by chemokine gradients such as CCR4-CCL17/22, CCR8-CCL1, CCR10-CCL28, and CXCR3-CCL9/10/11. Regulatory T cells are then activated and inhibit antitumor immune responses. A high infiltration by Treg cells is associated with poor survival in various types of cancer. Therefore, strategies to deplete Treg cells and control of Treg cell functions to increase antitumor immune responses are urgently required in the cancer immunotherapy field. Various molecules that are highly expressed by Treg cells, such as immune checkpoint molecules, chemokine receptors, and metabolites, have been targeted by Abs or small molecules, but additional strategies are needed to fine-tune and optimize for augmenting antitumor effects restricted in the TME while avoiding systemic autoimmunity. Here, we provide a brief synopsis of these cells in cancer and how they can be controlled to achieve therapeutic outcomes.
Keywords: Treg; immune checkpoint; immune suppression; tolerance; tumor.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
No potential conflict of interest was disclosed by YO. HN has received honoraria and grants from Bristol‐Myers Squibb, Chugai, and Ono and grants from Astellas, BD Japan, Daiichi Sankyo, Kyowa Hakko Kirin, Sysmex, Taiho, Asahikasei, and Zenyaku Kogyo.
Figures
References
-
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self‐tolerance. Cell. 2000;101:455‐458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

