Insulin Glargine Dose and Weight Changes in Underweight, Normal Weight, and Overweight Children Newly Diagnosed with Type 1 Diabetes Mellitus
- PMID: 31102482
- PMCID: PMC6609461
- DOI: 10.1002/phar.2281
Insulin Glargine Dose and Weight Changes in Underweight, Normal Weight, and Overweight Children Newly Diagnosed with Type 1 Diabetes Mellitus
Abstract
Study objective: Newly diagnosed pediatric patients with type 1 diabetes mellitus (T1D) can be underweight, overweight, or normal weight at presentation. Study objectives were to determine if, across weight categories, admission body weight (ABW)-based initial insulin glargine dosing resulted in similar fasting blood glucose responses on day of discharge, how initial ABW-based doses differed from doses at outpatient follow-up, and whether an ideal body weight (IBW) would provide a better estimate of body weight after discharge.
Design: Retrospective chart review.
Setting: Urban tertiary academic medical center.
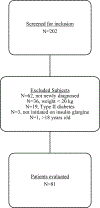
Patients: Eighty-one pediatric patients newly diagnosed with T1D who started therapy with subcutaneous insulin glargine between October 2014 and October 2016; patients were categorized by weight using body mass index (BMI) percentiles (underweight, normal weight, or overweight/obese).
Measurements and main results: Data on patient parameters from hospitalization to outpatient physician follow-up were collected. The McLaren, Moore, and BMI IBW methods were used to calculate IBW for each patient; these IBWs were compared with weights at outpatient follow-up. Initial insulin glargine doses were similar among all weight groups: median (range) 0.299 (0.227-0.4), 0.297 (0.204-0.421), and 0.291 (0.194-0.394) units/kg/dose, respectively, for the underweight, normal weight, and overweight/obese groups. No significant differences in discharge fasting glucose level or insulin glargine dose change from admission to first outpatient follow-up visit were noted. Underweight patients gained significantly more weight within 60 days after discharge compared with normal and overweight/obese patients, (median 16.3% vs 7.7% and 5.7%, respectively; p=0.002), aligning closest with the McLaren IBW. ABW was the best estimate of weight at outpatient follow-up in the overweight/obese patient group.
Conclusion: For children who presented underweight, the McLaren IBW method was the best predictor of outpatient dose and body weight, whereas ABW was the best estimate in overweight and obese patients. Further investigation of the role of IBW- or ABW-based dosing methods in underweight pediatric patients with T1D may assist in optimal dosing.
Keywords: ideal body weight; insulin glargine; pediatrics; type 1 diabetes mellitus.
© 2019 Pharmacotherapy Publications, Inc.
Conflict of interest statement
Declaration of Conflicting Interests
The authors report no relevant financial relationships and have no conflicts of interest to disclose.
Figures
References
-
- Bacha F, Klinepeter Bartz S. Insulin resistance, role of metformin and other non-insulin therapies in pediatric type 1 diabetes. Pediatr Diabetes 2015;17(8):545–558. - PubMed
-
- Wiegand S, Raile K, Reinehr T, et al. Daily insulin requirement of children and adolescents with type 1 diabetes: effect of age, gender, body mass index and mode of therapy. European Journal of Endocrinology 2008;158(4):543–549. - PubMed
-
- Statistics About Diabetes. American Diabetes Association 2018. Available at: http://www.diabetes.org/diabetes-basics/statistics/. Accessed April 3, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical