Strategies, design, and chemistry in siRNA delivery systems
- PMID: 31102606
- PMCID: PMC6745264
- DOI: 10.1016/j.addr.2019.05.004
Strategies, design, and chemistry in siRNA delivery systems
Abstract
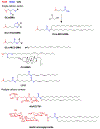
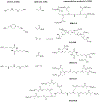
Emerging therapeutics that utilize RNA interference (RNAi) have the potential to treat broad classes of diseases due to their ability to reversibly silence target genes. In August 2018, the FDA approved the first siRNA therapeutic, called ONPATTRO™ (Patisiran), for the treatment of transthyretin-mediated amyloidosis. This was an important milestone for the field of siRNA delivery that opens the door for additional siRNA drugs. Currently, >20 small interfering RNA (siRNA)-based therapies are in clinical trials for a wide variety of diseases including cancers, genetic disorders, and viral infections. To maximize therapeutic benefits of siRNA-based drugs, a number of chemical strategies have been applied to address issues associated with efficacy, specificity, and safety. This review focuses on the chemical perspectives behind non-viral siRNA delivery systems, including siRNA synthesis, siRNA conjugates, and nanoparticle delivery using nucleotides, lipids, and polymers. Tracing and understanding the chemical development of strategies to make siRNAs into drugs is important to guide development of additional clinical candidates and enable prolonged success of siRNA therapeutics.
Keywords: Nanomaterials; Therapeutics; siRNA.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures







References
-
- Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998). - PubMed
-
- Garber K Alnylam launches era of RNAi drugs. Nat Biotechnol 36, 777–778 (2018). - PubMed
-
- Robb GB & Rana TM RNA helicase A interacts with RISC in human cells and functions in RISC loading. Mol Cell 26, 523–537 (2007). - PubMed
-
- Gaynor JW, Campbell BJ & Cosstick R RNA interference: a chemist’s perspective. Chem. Soc. Rev 39, 4169–4184 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

